Introduction
High levels of the fraction of inspired oxygen (FiO2) and partial pressure of oxygen (PaO2) are commonly used in clinical practice for patients with acute respiratory failure (ARF) and other conditions to reverse hypoxemia.1–3 However, these patients are at greater risk of hyperoxia, which can have deleterious effects on the body. These effects include damage to the lung parenchyma due to free oxygen radicals, activation of apoptosis pathways, atelectasis, increased production of pro- and anti-inflammatory cytokines, increased polymorphonuclear and alveolar-capillary “leak” resulting from tissue fibrosis, increased vascular resistance, decreased cardiac output, and reduced myocardial, renal and cerebral blood flow.2,4,5
A cohort study conducted by Helmerhorst et al. revealed an increase in mortality and the duration of mechanical ventilation (MV) resulting from hyperoxia.6 Similarly, a clinical trial by Girardis et al. demonstrated an increase in mortality related to liberal oxygen therapy (PaO2 until 150 mmHg or SpO2 97 to 100%) compared with conservative oxygen therapy (PaO2 = 70 to 100 mmHg or SpO2 94 to 98%), with lower levels of FiO2 and PaO2.7 However, other clinical trials have not shown a significant difference in mortality with the use of both types of oxygen therapy.8–11 Although several systematic reviews have explored this topic, most included a small number of studies and combined data from heterogeneous populations and observational designs.12–15 Consequently, the true impact of oxygen therapy type on clinically relevant outcomes remains uncertain. An updated, methodologically rigorous synthesis restricted to randomized controlled trials is therefore warranted to clarify these effects and guide evidence-based clinical practice. Therefore, the aim of this study was to conduct a systematic review with a meta-analysis that summarized the evidence on the use of conservative oxygen therapy compared with liberal oxygen therapy for patients hospitalized on mechanical ventilation.
Methods
This is a systematic review with a meta-analysis in which the criteria established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines of 2020 were used.16 The protocol was published in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42021235802.
Information sources and search strategy
The literature search was carried out in the MEDLINE/PubMed, Embase, Cochrane Library, Lilacs/bvs, PEDro and ScienceDirect databases through a combination of descriptors and Boolean operators, including terms from Medical Subject Headings (MeSH), Emtree and Descriptors in Health Sciences (DeCS). The search strategy (Supplementary material, Table 1) was built according to the PICO acronym (population, intervention, comparison and outcomes).
Eligibility criteria
Only randomized controlled trials (RCTs) that compared conservative oxygen therapy with liberal oxygen therapy for mortality in patients on MV (invasive and noninvasive mechanical ventilation) in the intensive care unit and aged 18 years or older were included. Furthermore, controlled and randomized clinical trials were eligible for inclusion in this systematic review, without any restrictions regarding publication year or language.
Two different strategies exist for supplemental oxygen in acutely ill patients: high-target (liberal) or low-target (conservative) oxygen therapy. However, seemingly arbitrary variations in thresholds of oxygenation exist for these two strategies in research studies. In the present study, for the purpose of conducting the meta-analysis, oxygen therapy was categorized into lower O2 (PaO2 ≤ 60 and/or SpO2 < 90%), intermediate O2 (PaO₂ 61–119 mmHg and/or SpO₂ 90–95%), and higher O2 (SpO2 ≥ 96% or PaO2 ≥ 120 mmHg or higher FiO2 administered) on the basis of the available guidelines. Studies that used only noninvasive ventilation and/or high-flow nasal cannulas were excluded.
Data collection process and data items
The identification and selection of studies were carried out independently by two reviewers (IGNA and GFV). This process involved reading the title and abstract of the studies found in the search. Eligible articles were then selected on the basis of the inclusion and exclusion criteria and read in full. In the event of any discrepancy between the results found by the two reviewers, a third reviewer (SFOJ) was consulted to resolve them. The articles were organized and read via Zotero version 6.
The data were extracted and synthesized into an Excel table. The recorded characteristics of the studies included the title, author, year of publication, study design, language, country of origin, periodical, sample size, mean age (with standard deviation), type of outcome, objective of the study, research protocol for oxygen therapy, follow-up time, results found and conclusion.
Study risk of bias assessment and certainty assessment
The risk of bias analysis was independently conducted by two reviewers, who used the risk of bias tools (RoB2 and RoB2 for cluster-randomized trials). The risk of bias was assessed according to the following domains: 1) The randomization process; 2) Deviations from intended interventions; 3) Missing outcome data; 4) Measurement of the outcome; and 5) Selection of the reported results. Any disagreements were resolved by discussion or by involving another review author. The quality of evidence analysis was performed via the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.17 In cases of disagreement, a third reviewer participated in both analyses.
Synthesis methods and effect measures
In the meta-analysis, the effect estimates for mortality were demonstrated as relative risk. For the other variables, the combined effect estimates were expressed as the mean difference between the groups. Statistical heterogeneity was assessed via the Cochran Q test and the I² test, in which values above 40% were considered moderate to high heterogeneity.18 Effect measure calculations were performed via a fixed or random effect model, taking into account the heterogeneity of the pooled data. Two sensitivity analyses were conducted to investigate the potential effects of certain studies on heterogeneity and the overall effect. The first subgroup analysis separated studies that included patients undergoing invasive mechanical ventilation (IMV) from studies with patients on both IMV and noninvasive ventilation (NIV), whereas the other subgroup analysis separated studies that classified the oxygen profile as lower O2 versus intermediate O2 versus higher O2. A significance level of 0.05 was considered. The analysis was conducted via Review Manager version 5.3 (Cochrane Collaboration). It is noteworthy that, of the 19 studies included in the review, only 17 reported at least one of the outcomes in a quantitative format—specifically as a mean or mean difference, accompanied by a measure of dispersion and sample size—thereby enabling their inclusion in the meta-analyses.
Results
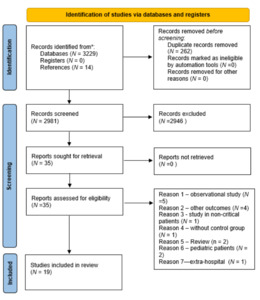
Initially, a total of 3,229 articles were identified in the search. Additionally, 14 studies were identified through other means, such as searching for citations, resulting in the identification of a total of 3,243 articles. After removing duplicates and excluding articles on the basis of the title and abstract, 35 articles remained. After the eligibility criteria were analyzed, 19 studies were included in the review (Figure 1).
Characteristics of the included studies
The 19 studies included (N = 15,238) are controlled and randomized clinical trials that aim to evaluate the effects of liberal oxygen therapy compared with conservative therapy for the following outcomes: 1) mortality7–11,19–30; 2) length of stay in the ICU7,9,11,20–22,24–26,31; 3) ventilation-free days or hours7,9,11,22,26,29 and 4) PaO2/FiO2,21,26,32 as described in (Supplementary material, Table 2).
The average age of the included patients was 60.3 (±8.49) years, and 63.5% were men. The most prevalent comorbidities in the sample were chronic obstructive pulmonary disease (COPD),7,10,11,24,25 cancer,7,10,11,22,24 heart disease,10,11,20,24 cardiac arrest,27 SIRS28 and kidney failure.7,11,23,32 Other comorbidities included acute respiratory distress syndrome (ARDS),8,21 stroke,9,24,32 coronary artery disease,10,11,20,24 diabetes,21,24,32 and hypertension.20,24,25,32 The oxygen therapy protocol used for each study group is described in Supplementary material, Table 2.
Risk of bias in studies
The results of ROB 2 or ROB 2 for cluster randomized trial analysis are shown along with the presentation of the meta-analyses.
GRADE: certainty of evidence
The certainty of evidence using the GRADE comparison was moderate to very low for the selected outcomes. We downgraded the quality of evidence ratings predominantly owing to inconsistency, imprecision, and risk of bias (Supplementary material, Table 3).
Qualitative synthesis
Adverse Events
Most studies did not quantify adverse events related to different oxygen supplementation protocols. Those who assessed this outcome did not find statistically significant differences in the occurrence of adverse events among the protocols, with the exception of the ICU-ROX study. Thus, in the ICU-ROX study, one patient in the conservative-oxygen group experienced hypoxemia with a PaO2 of 33.5 mmHg, and a second patient had a low SpO2, although the exact value was not recorded. Furthermore, one patient in the usual-oxygen group experienced ischemic stroke, which was also categorized as an adverse event.
On the other hand, Lang et al. emphasized the absence of any adverse effects of hyperoxia on the lungs.21 In the study by Panwar et al., there were no major adverse events associated with a conservative oxygenation strategy.26 In the study by van der Wal et al., severe adverse events (SAEs) were defined as follows: PaO2 < 37.5 mmHg and SpO2 < 80%.30 Consequently, SAEs occurred in 13 (3.9%) patients in the low-O2 group, and 22 (6.7%) adverse events were observed in the high-O2 group. Ischemia was observed in 10 patients in the lower-O2 group and in 15 patients in the higher-O2 group. Cardiac arrest was identified in two patients in the lower-O2 group and in four patients in the higher-O2 group. No patient exhibited PaO2 < 37.5 mmHg; however, one patient in the low-O2 group and two patients in the high-O2 group had SpO2 < 80%.
Sepehrvand et al. demonstrated the absence of significant differences in the occurrence of adverse events between the two groups with oxygen supplementation.24
In the ICU-ROX study, one patient allocated to the conservative oxygen therapy arm experienced an adverse event due to inadvertent exposure to hypoxemia. In the study by Barrot et al., the occurrence of infectious, neurological, and cardiovascular adverse events did not differ between the groups (arrhythmia: 23.2% in the conservative oxygen group and 15.7% in the liberal oxygen group; septicaemia: 11.1% in the conservative oxygen group and 18.6% in the liberal oxygen group).8
In Schjørring et al., one or more serious adverse events, defined as a new episode of shock, myocardial ischemia, cerebral ischemia, or intestinal ischemia, were reported.10 Similarly, the number of patients with one or more serious adverse events did not differ significantly between the two groups (shock, 33.9% vs 35.8%; myocardial ischemia, 1.0% vs 0.5%; ischemic stroke, 1.3% vs 1.6%; intestinal ischemia, 2.0% vs 2.2%).
Additionally, the most frequent adverse events were infection, bleeding, and seizures. There were no significant between-group differences in any prespecified adverse events.27 Acute kidney failure developed in 20 patients (10%) in the low-normal PaO2 group and 21 patients (11%) in the high-normal PaO2 group, and acute myocardial infarction developed in 6 patients (2.9%) in the low-normal PaO2 group and seven patients in the high-normal PaO2 group (3.6%).28
Finally, in the study by Jakkula et al., adverse events that could be related to the interventions included severe hypercapnia and respiratory acidosis (PaCO2 > 75 mmHg and pH < 7.15).20 No difference was observed between the groups (normoxic and moderate hyperoxic).
Quantitative synthesis
Mortality
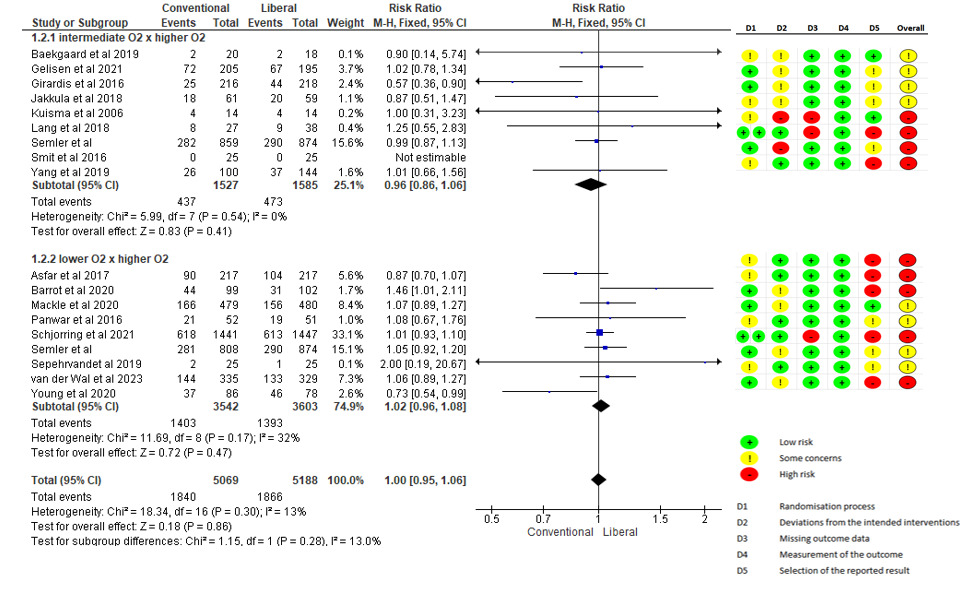
Seventeen of the studies included in this review evaluated mortality outcomes (24 h, 48 h, 28 days, 30 days, 90 days, 180 days and hospital or ICU mortality). Thus, when combined, the meta-analysis revealed the absence of a significant difference in this outcome when the group receiving conservative oxygen therapy was compared with the group receiving liberal oxygen therapy (RR 1.00 - 95% CI: 0.92 to 1.07; N = 8661, moderate GRADE), as shown in Figure 2. In the subgroup analysis with studies that included patients on IMV and with studies that combined IMV and NIV (Figure 3), there was also no statistically significant difference in mortality in either the subgroup with IMV (1.00 - 95%: 0.92 to 1.07; N = 4806, moderate GRADE) or the subgroup with IMV and NIV (RR 1.00 - 95%: 0.78 to 1.29; N = 3855, moderate GRADE). Additionally, a meta-analysis was conducted, including eight studies that analyzed 90-day mortality (two studies IMV plus NIV and six studies IMV only), and no significant difference was detected between the groups (Supplementary material, Figure S1).
Another subgroup analysis was performed to classify the studies on the basis of the oxygen supply profile between the groups. The studies were divided into those that used lower O2 versus intermediate O2 versus higher O2 groups and those that used normoxia versus hyperoxia groups (Supplementary material, Table 4). This classification was based on the review by Cumpstey et al.15 The results revealed no statistically significant difference in mortality between the normoxia (intermediate O2) and hyperoxia (higher O2) subgroups (RR 0.96 –95%: 0.86–1.08; N = 3.112, moderate GRADE) or between the hypoxia (lower O2) and hyperoxia (higher O2) subgroups (RR 1.02–95%: 0.96–1.08; N = 2.796) (Figure 2). In the subgroup analysis of patients with ARDS, there was also no statistically significant difference in mortality between the conventional and liberal oxygen therapy subgroups (RR 1.04–95%: 0.97–1.12; N = 3856, moderate GRADE) (Figure 4).
Length of stay
Two studies were included in the analysis of length of stay, and the meta-analysis revealed that there was no significant difference in this outcome (MD 0.18 - 95% CI: - 2.69 to 3.05; N = 502, GRADE very low) when the conservative oxygen therapy group was compared with the liberal oxygen therapy group (Figure 5).
Days free from mechanical ventilation
To analyze the outcome of mechanical ventilation-free days, three studies were included. The meta-analysis (Figure 6) revealed no significant difference in this outcome (MD 0.25, 95% CI: -1.78–2.27; N = 1496, GRADE very low) when the conservative oxygen therapy group was compared with the liberal oxygen therapy group.
.
Discussion
This systematic review included 19 controlled and randomized clinical trials comparing liberal versus conservative oxygen therapy in patients receiving mechanical ventilation, of which 17 RCTs were included in the meta-analysis. The results revealed no statistically significant differences in mortality (moderate degree of evidence), length of stay in the ICU (very low level of evidence), or duration of MV (very low level of evidence) between the groups. Subgroup analysis was also conducted for mortality, separating studies with patients exclusively on IMV from those with a combination of IMV and NIV, as well as studies that included patients with ARDS, possibly because there may be a difference in the severity profile of these patients, as invasive surgery is indicated for more severe or refractory cases of ARF.1,33 However, no difference was found between the groups in these analyses.
This study is one of the first systematic reviews with meta-analyses to include only controlled and randomized clinical trials (RCTs) available in the literature. Other systematic reviews have also reported no difference in mortality between groups.12–15,34 However, these reviews have limitations, such as the inclusion of observational studies and pediatric patients, as well as the exclusion of recently published studies. These limitations reduce the confidence in the findings of previous reviews, which justifies the need for the present review. Importantly, despite the methodological rigour of this review, the presence of heterogeneity and the considerable risk of bias still resulted in a low or very low level of evidence for the findings (Supplementary material, Table 3).
In clinical practice, it is common to use oxygen therapy on the basis of patients’ complaints or signs of dyspnea rather than relying solely on oxygen saturation levels. However, this approach can often lead to overexposure to oxygen and its associated negative effects. This review supports the notion that liberal oxygen therapy is not superior to conventional oxygen therapy and may not be justified in clinical practice. Therefore, the use of liberal oxygen therapy may not be justified in clinical practice. Additionally, this review revealed no evidence to support the superiority of conventional oxygen therapy in patients with ARDS. Thus, there is no justification for intentionally inducing hypoxia (lower O2) in these patients, as it does not reduce ICU mortality.
Importantly, there were challenges in defining the parameters of hyperoxia (higher O2), normoxia (intermediate O2), and hypoxia (lower O2) for each included randomized clinical trial. The heterogeneity in the profiles of the means and standard deviations of SpO2 and PaO2 between the intervention and control groups in individual studies may have contributed to potential differences between the groups not identified in the analysis. Therefore, future studies on this topic should define normoxia (intermediate O2), hypoxia (lower O2), and hyperoxia (higher O2) on the basis of PaO2 and SpO2 values rather than FiO2. This is necessary because different severity levels among patients can result in different PaO2 and SpO2 values even with the same programmed FiO2.
Notably, the included studies presented heterogeneous samples in size and comorbidities. There was also an analysis of different follow-up points within the same study and between them.7–9,11,19,22–24,26,32 Furthermore, half of the studies included in the present review presented a high risk of bias8,9,11,21–23,29,32 (Rob2 or Rob2 for cluster-randomized trials). This is because some of them did not maintain allocation confidentiality, and there was interference by the medical team in the allocation of patients between the groups. Therefore, we conducted a detailed and consistent analysis of the risk of bias and the level of evidence.
Considering that there is biological plausibility in the hypothesis that the supply of excess O2 can be harmful to the body and lead to greater complications in the clinical care, the possibility of clinically significant differences between interventions cannot be excluded.4,6,35–39
The strengths of this review were the comprehensive search and selection of articles, including exclusively randomized controlled clinical trials. Additionally, the protocol for this study was previously registered, and the level of evidence was analyzed for each outcome via the GRADE. Another important point was the subgroup analysis that was conducted, which provides greater sensitivity to the results obtained. Our main contribution to the literature is the present review, which is methodologically consistent with important findings regarding the different oxygen therapy protocols used in MV patients.
Conclusion
This systematic review with meta-analysis revealed that the use of liberal oxygen therapy did not result in a reduction in mortality, length of stay or mechanical ventilation. Therefore, the use of liberal oxygen therapy should not be recommended. Similarly, for patients with ARDS, there was no difference in mortality when oxygen therapy aimed at maintaining hypoxemia levels to maintain oxygenation in a normoxic range was compared. Importantly, however, the included studies had a high risk of bias, and the level of recommendation for outcomes such as length of hospital stay and duration of mechanical ventilation was low or very low. Therefore, there is a need for new clinical trials with better methodologies to provide stronger statistical and clinical evidence. Additionally, more controlled clinical trials are needed with well-defined parameters for oxygen therapy at different levels (lower, intermediate, and high) for comparisons between groups.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
All authors have completed the ICMJE uniform disclosure form and declare no conflict of interest.
Ethical Approval
Not required for this article type. The study was registered with PROSPERO: number CRD42021235802.
AI Statement
The authors confirm that no generative AI or AI-assisted technology was used to generate content.