Introduction
Pulmonary rehabilitation (PR) programs often include an educational component. Its purpose is to improve the knowledge and skills of patients and their families for the care and autonomous management of their chronic respiratory pathology and reinforce adherence to physical exercise.1,2 Chronic Obstructive Pulmonary Disease (COPD) is defined by the long-term obstruction of airflow, causing chronic cough, sputum production, dyspnea, and exacerbations.3 Implementing educational interventions in COPD patients has shown significant reductions in the probability of hospital admission for exacerbations, improvement in health-related quality of life (HRQoL), and the perception of dyspnea.4,5
COPD patients who complete PR experience improved exercise tolerance, dyspnea control, and overall quality of life, enabling them to lead a more active lifestyle.5 However, a considerable number of COPD patients fail to complete PR programs, forfeiting the benefits6 and imposing a higher financial burden on healthcare systems.7 Additional tactics, like education, may prove helpful in maintaining the benefits of PR and ensuring compliance with health provider guidance.8
Telephone follow-up (TFU) is a strategy that allows continuous communication with the patient beyond face-to-face encounters and has demonstrated positive effects on adherence to PR treatment.9–12 However, there is limited research studying the effects of including TFU as part of the PR treatment to improve adherence and outcomes.
This study aimed to determine the effect of TFU on educational needs, dyspnea, quality of life, and functional capacity in COPD patients undergoing PR.
Methods
A double-blinded, randomized clinical trial was conducted on patients with COPD who participated in a PR program at a clinic in Cali, Colombia, from February to August 2020.
The clinic’s ethics committee approved this study, adopted all the recommendations of the Declaration of Helsinki and Resolution 008430 of the Colombian Ministry of Health and Social Protection, and was approved by the ethics committee of the National School of Sports (Cali-Colombia) Act #126.01.05.03 / May 12, 2020, and got Clinical Trials code NCT05204498. All participants voluntarily consented by signing the informed consent form.
Inclusion criteria: having a diagnosis of COPD confirmed by post-bronchodilator spirometry and pulmonologist taking into account the Global Initiative for Chronic Obstructive Lung Disease (GOLD) subgroup classification (A, B, C, D)3; being between the ages of 50 and 80 years; starting for the first time and completing the PR program and completing at least 85% of the educational sessions. Exclusion criteria: having experienced worsening cardiovascular and metabolic disease leading to hospital or emergency room visits within the past month. Patients receiving treatment with corticosteroids during the previous month and presenting cognitive impairments that restricted their participation in educational sessions.
The Armitage & Berry formula was chosen to calculate the sample size, allowing determining the sample size to find the difference between two independent means.13 The calculation was made with the following data: precision 1.96, power 0.842, standard deviation 1.23, and magnitude of the expected difference 1.19. The required sample size was 16.78 for each group.
The randomization of patients into the intervention groups was carried out once they had completed their consultation with a specialist physician, who verified the inclusion criteria for admission to PR. The patients were then sent to an external person who used the Microsoft Excel program to list the boxes in a table to which a simple randomization formula was applied. Thus, the patients were registered in numerical order and automatically classified in the group they belonged to according to the randomization. The results were sealed in a manila envelope and delivered to an educator trained in education and TFU, who was not part of the research team and was responsible for making the TFU for participants after the PR sessions. Subsequently, they were assigned the schedule and sessions of the PR. This study was double-blinded from the principal investigator and the health professionals participating in the PR program.
Procedures
A physiotherapist specializing in cardiopulmonary rehabilitation evaluated the participants at the beginning of PR and after eight weeks of intervention.
Sociodemographic and clinical data were collected, such as age, sex, socioeconomic status, marital status, home oxygen use and body mass index (BMI), Forced Vital Capacity (FVC), Forced Expiratory Volume in the first second (FEV1), and the ratio (FEV1/FVC) from spirometry (American Thoracic Society ATS: Standardization of Spirometry).14
The Lung Information Needs Questionnaire (LINQ) was used to assess educational needs and knowledge of the disease. This questionnaire includes 19 questions, 16 closed multiple-choice questions, and three open-ended questions, one related to doubts about the disease and the other two with demographic data. The questions are grouped into six domains. The maximum score obtained is 25 and is related to a greater need for education; the minimum score obtained is 0.15,16
The patients self-administered the HADS (Hospital Anxiety and Depression Scale) questionnaire,17 of which has 14 items divided into two subscales (anxiety and depression), each with seven questions. Scores greater than or equal to 11 points reflect clinical problems, scores between 8 and 10 are considered doubtful or risky, and scores lower than seven are standard.
The modified Medical Research Council (mMRC) dyspnea scale was used, with scores ranging from 0 to 4, where 0 represents the absence of dyspnea except during intense exercise and increases to 4, a score that refers to the presence of dyspnea that prevents the patient from leaving the house or that appears even with activities such as dressing.18
The Saint George’s Respiratory Questionnaire (SGRQ) allowed the evaluation of HRQoL. That questionnaire has 50 questions grouped into three dimensions: symptoms, activity, and impact; scores range from 0 to 100, with values close to 0 indicating better HRQoL.19 A decrease of 4 points means a clinically significant improvement.20
The questionnaires were chosen for their frequent use and familiarity in hospital and non-hospital settings by healthcare professionals and patients.
Functional capacity was measured with the 6-minute walking test (6MWT) using ATS recommendations.21 The best distance covered by the two tests performed was taken, as well as the estimated peak oxygen consumption (VO2e) calculated at VO2e=3.5 ml/kg/min + (velocity m/min × 0.1).22
Pulmonary Rehabilitation Program
The PR program included 24 sessions, carried out three times per week for eight weeks. Each session comprised continuous exercise on a treadmill or ergometric bicycle for 30 minutes, starting at 60% of the VO2e reached in the 6MWT. Progression in intensity was performed using the modified Borg scale, increasing until scores between 3/10 and 5/10 were reached.23 Muscle-strengthening exercises were performed in four series of 12 repetitions, starting at 50% of maximum resistance (MR) and increasing until reaching scores between 3/10 and 5/10. The estimation of the MR was made considering the highest number of times that the patients were able to perform each movement evaluated using the correct technique.24 Exercise sessions lasted 60 minutes.
Patients who had saturation <90% or desaturation ≥ 4 points from baseline saturation during the 6MWT were administered oxygen to maintain SpO2 ≥90% during exercise sessions.23
Educational Program
The control group, called PR plus traditional education (PRTE), received group and individual education by health professionals linked to the PR program on topics related to knowledge of the disease, the importance of quitting smoking, use of inhalers, recognition of warning signs, use of home oxygen, proper nutrition, energy conservation techniques, and home breathing exercises.25 During the PR, each participant received individual education for 30 minutes per week regarding topics as previously mentioned. Additionally, the whole group of participants received 60 minutes of group education per week. Patients could attend in the company of a family member; in these sessions, patients had the opportunity to share their experiences and questions about managing their disease with the other attendees and with the professional in charge who moderated the session and resolved the participants’ concerns.
The intervention group—called PR, traditional education, and educational telephone follow-up (PRTETFU)—received the same intervention as the control group. However, an experienced health professional outside the PR program provided additional education to patients with chronic respiratory disease over the telephone. The calls lasted 10-15 minutes and aimed to complement the topics discussed during the face-to-face PR program. The phone calls were conducted twice weekly between 8:00 a.m. and 11:00 a.m. over eight weeks. To ensure consistency of information delivery, pre-designed scripts were utilized to deliver information on chosen topics to all patients (see Supplementary information).
Statistical Analysis
The information was recorded in Microsoft Office Excel® 2010 and analyzed in the SPSS v24 statistical package. The sociodemographic variables are presented in frequencies and percentages; normality tests were performed for each quantitative variable with the Shapiro-Wilk test, presenting the variables with normal behaviour as mean ± standard deviation and the variables that did not comply with normality as median and interquartile range. A t-test for independent samples was performed to compare the variables between each group before and after. The t-test for related samples was performed to compare differences in means and the Wilcoxon test to compare differences in medians at each group’s end of the PR program; a p-value <0.05 was considered statistically significant.
Results
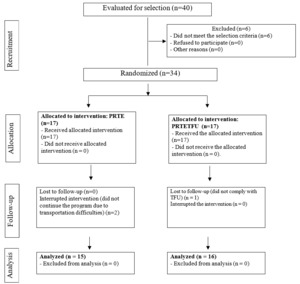
Eligibility for the clinical trial was assessed in 40 patients referred for PR for six months (February to July 2020). Six patients were excluded because they had previously undergone PR, and 34 patients were enrolled in the trial and randomly assigned to the PRTE and PRTETFU groups. There was a dropout rate of 5.5% (two patients who could not transport themselves to the clinic) in PRTE and 3.1% (one patient who did not complete 85% of the educational sessions) in PRTETFU (Figure 1).
At the beginning of the study, baseline variables had no significant differences between groups (Table 1). The mean age was 65.5 years (±13.69), primarily men (71%) with a history of smoking (64.5%) and had consulted the emergency department for an increase in symptoms in the last year (83.9%); regarding clinical characteristics, the Tiffeneau Index indicated a mean of 56.5 ±13.0 percent and BMI an average of 25.6 ±4.3 kg/m2, reflecting an overweight population (Table 1).
Regarding aerobic capacity, the mean distance travelled in the 6MWT was 349.5m ± 107.5, dyspnea assessment was 3.03 ± 1.02 according to mMRC, and 32.3% had an indication for home oxygen. Patients reported the presence of needs in education with a total score of 11.0 ± 4.1 on the LINQ. In the HADS questionnaire, no anxiety or depression symptomatology was observed, and HRQoL had a mean score of 53.3 ± 17.3 (Table 2).
There were significant improvements in the experimental group (PRTETFU) in all LINQ domains (knowledge p=0.001, medications p=0.006, self-care p=0.000, exercise p=0.005, diet p=0.048, and total score p=0.000) except in the smoking domain score. Similarly, there were improvements in distance travelled in the 6MWT with an increase of 62.8m ± 21.4 (p=0.010), and in dyspnea score, a decrease of 0.6 points ±0.3 (p=0.036). Regarding HRQoL, there was a statistically significant improvement in the activity domain (p=0.004) and clinically significant improvements for the remaining domains (symptoms and impact) and the SGRQ total score (Table 3).
In the control group (PRTE), there were significant improvements for the LINQ in the total score (p=0.003) and in only one of the domains, which was exercise (p=0.000). Likewise, greater distance travelled was observed in the 6MWT with an increase of 45.9 meters ± 16.1 (p=0.013) and a reduction in dyspnea score of 1.6 points ± 0.3 (p=0.000). Regarding HRQoL, there were improvements in the domains of symptoms (p=0.000) and impact (p=0.006), and in the SGRQ total score (p=0.004), the activity domain did not show significant improvements; on the contrary, there was a deterioration of 8.3 (±8.1) points (Table 3).
At the end of the study, a statistically significant improvement was found in the LINQ knowledge domain score in favour of the PRTETFU patients. Significant differences were found in the SGRQ impact domain while the remaining outcome measures were analyzed. The control group (PRTE) scored 23.3 points lower (±16.3) compared to the experimental group (PRTETFU), which scored 40.9 points higher (±23.1) with a p-value of 0.021 (Table 4).
Discussion
This study aimed to determine the effect of TFU on educational needs, dyspnea, quality of life, and functional capacity in COPD patients undergoing PR. Our results suggest that an educational component added to aerobic and strength exercises in a PR program improves knowledge, aerobic capacity, dyspnea, and HRQoL for individuals with COPD. Additionally, providing educational support through telephone calls improves patients’ understanding and abilities to manage their illness.
All patients in this study improved their knowledge of their disease and self-care strategies, with significantly better results in the experimental group (PRTETFU). These results align with those previously reported by other researchers.26 For example, García-Aymerich et al. developed self-care educational reinforcement by telephone. They found significant improvements in symptom knowledge (p=0.005) and treatment adherence (p=0.009), with no changes in other measures such as dyspnea, lung function, and HRQoL. Walters et al.,27 also observed improvements in self-care capacity but no impact on HRQoL nor symptoms of anxiety and depression. Nevertheless, the educational interventions proposed by the studies above were developed outside the framework of a PR program.
Other studies have used telephone communication as a strategy to monitor COPD control. Some through daily monitoring technology platforms connected directly to the home telephone,28 monthly telephone follow-ups on symptoms and exacerbations,29 or assignment of individualized comprehensive care plans with telephone reinforcement to ensure adherence.30 Among the most relevant findings are significant improvements in HRQoL,28 health care costs, timely identification of exacerbations, reduction of hospital admissions,29 and recovery time after an exacerbation.30 However, none of these telephone communication strategies were part of a PR program.
The studies that include telephone support during PR are generally for home PR programs or investigate treatment adherence several months after completion. While this study reports similar results regarding adherence to the PR,31 the outcomes from other studies utilizing telephonic support are somewhat contradictory. There were positive impacts in one of the studies: less dyspnea, more exercise tolerance, and improvement in HRQoL in those who received home PR with TFU (p <0.05)32; the opposite was shown in the study by Hornikx et al., in which there were no significant differences in physical activity in patients who had telephone counselling to guide home physical exercise.33 Similarly, regarding the impact of long-term PR, Wong et al. showed that providing telephone support six months after completing PR was no more effective than usual care in maintaining health outcomes, specifically, HRQoL and functional capacity measured with the 6MWT.34 Our study presents unique findings because the telephone guidance stressed the importance of exercising in a planned, organized, and standardized manner rather than just engaging in physical activity to increase energy expenditure.
The most recent systematic review with meta-analysis that evaluated the impact of telephone support on the quality of life and exercise capacity of COPD patients included studies that used telephone calls as a strategy to facilitate adherence, monitor symptoms, achieve disease control after an exacerbation, or support home-based PR. However, none of them investigated telephone follow-up as a strategy to reinforce the educational component provided in a personalized manner during PR. However, none of them investigated telephone follow-up as a strategy to reinforce the educational component provided in a personalized manner during PR, as did our study. The main finding of this meta-analysis is the effect of telephone-based interventions achieving statistically significant improvements in the SGRQ scores (p <0.00001) but not in the 6MWT.35
Regarding HRQoL results, all participants in this study demonstrated improvement on the SGRQ, some domains with statistically significant improvements and others with clinically significant improvements. However, it is noteworthy that the control group (PRTE) showed a deterioration of 8.3 ±8.1 points in the pre- and post-PR activity domain and, on the contrary, significant improvements in the impact domain when comparing the two groups at the end of the study. With regard to these results, it should be noted that it has been shown that the deterioration of HRQOL in COPD is not directly related to the increase in disease severity36 and that the answers to the SGRQ questions are expressions of the patient’s feelings at that moment and therefore may be influenced by his or her mood at the time of assessment,37 which could explain the contradictory results in the PRTE group.
As can be seen up to this point, education for self-management of COPD involves a process that should go beyond providing information, especially if it is considered that due to chronic hypoxemia and hypercapnia, aspects of cognitive capacity such as information processing, concentration, and memory are affected in these patients.38,39 Thus, including the use of strategies that effectively reinforce the message of self-management and that allow continuous communication with the patient beyond the PR session through technological tools or telephone calls, as in the case of the present study, are key to achieving better results in the management of COPD patients.
Although the results are interesting, it is essential to mention that the population was confined due to the COVID-19 pandemic in Colombia in March 2020. This fact limited the involvement of a larger population and could, to some extent, affect the perception of quality of life and mental and cognitive health status in some patients not considered for this study. In turn, the results cannot be generalized to the context of all PR programs. For further research, it is recommended to evaluate the long-term impact to identify whether the effects of the intervention can be sustained over time or if reinforcement of the education component is necessary as time passes. Additionally, the findings of this study could serve as a valuable contribution to developing educational strategies for younger patients within the context of PR programs.
Despite the limitations, our results show a significant improvement in knowledge for autonomous COPD management using an additional TFU during PR. Using a TFU is a simple and inexpensive procedure that can be easily replicated in other rehabilitation centers.
Conclusions
Education about the self-care of patients with chronic respiratory diseases should be an integral part of any PR program. This study suggests that the educational component provided in person during pulmonary rehabilitation can be reinforced through TFU with more significant results in knowledge and skills for the management of their pathology.
Competing interests
The authors have no conflicts of interest to declare.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributors
All authors contributed to the conception or design of the work, the acquisition, analysis, or interpretation of the data. All authors were involved in drafting and commenting on the paper and have approved the final version.
Ethical statement
The study was approved by the ethics committee of the National School of Sports (Cali-Colombia) Act #126.01.05.03 / May 12, 2020, Clinical Trials code NCT05204498.
AI statement
The authors confirm no generative AI or AI-assisted technology was used to generate content.