Introduction
Interstitial lung diseases (ILDs) are groups of diffuse parenchymal lung conditions with overlapping clinical presentation and radiological features. The 2013 Global Burden of Disease Study reported that ILDs were ranked 40th in relation to global years of mortality.1 The global incidence of ILDs ranged from 1 to 31.5 per 100,000 patients per year, and the prevalence ranged from 6.3 to 7.1 per 100,000.2 The diagnosis and treatment of ILDs require a multidisciplinary approach. Professions involved in the management of ILDs include but are not limited to, respirologists, radiologists and pathologists.3 The trajectory of ILDs is unpredictable; thus, prognostication may be challenging. Interstitial lung disease classification and terminology have been modified and updated over the past decade.4 The American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/Latin American Thoracic Association have collaborated to endorse practice guidelines for ILD management.3–6
Hypoxemia in ILDs consists of multiple physiologic derangements, including diffusion impairment, ventilation–perfusion mismatch, and abnormalities of the pulmonary vasculature leading to pulmonary hypertension.7,8 Chronic repetitive inflammatory processes and aberrant wound healing can lead to progressive destruction in alveolar units (i.e., fibrosis) and consequently limitations to oxygen diffusion from alveoli to capillaries.9,10 Consequently, oxygen supplementation is a main treatment approach in both acute and chronic respiratory failure patients. However, achieving targeted oxygen levels can be challenging. Acute exacerbations of ILDs have been reported to be the most common causes of respiratory deterioration and are associated with poor outcomes. Specifically, acute exacerbation of ILDs accounts for 29-55% of respiratory hospitalizations and 20-33% of lower respiratory tract infections.11,12 ILD patients who require mechanical ventilation generally have poor outcomes due to the irreversible nature of their disease.13–17 Although extracorporeal membrane oxygenation (ECMO) is stated as a rescue modality in ILDs with refractory hypoxemia, it is unable to alter the mortality, particularly when patients do not qualify for lung transplantation.18
Current practice guidelines reflect certain favourable outcomes associated with noninvasive positive pressure ventilation (NIPPV) or high flow nasal cannula (HFNC) in selected acute respiratory failure (ARF) patients.19–21 Although ILDs were not included in those recommendations, primary studies suggest that NIPPV or HFNC might be potential approaches in this subgroup.22–24 The common ventilatory settings of NIPPV used in acute and/or chronic respiratory failure are known as Continuous Positive Airway Pressure (CPAP) and Bilevel Positive Airway Pressure (BiPAP).21 This review synthesizes current studies that compared the effectiveness of noninvasive respiratory supports (NIPPV and/or HFNC) and conventional oxygen therapy (COT) for improving oxygenation and ventilation in ILD patients with ARF.
Method
Eligibility criteria
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.25 We applied the following inclusion criteria: experimental studies (randomized, quasi-randomized, prospective and retrospective trials) that examined the benefit of NIPPV or HFNC on patients with ILDs and ARF and/or distress; adults (aged ≥ 18 years. The exclusion criteria were case reports and case series. The outcome measures included improvements in oxygenation and ventilation at 24 h or as defined by the study, using PaO2/FiO2 (PF ratio) and PaCO2, respectively; mortality; intubation rate; hospital length of stay and complications.
Information sources and search strategy
Systematic literature searches were conducted for studies published from inception to August 6, 2023, in MEDLINE, EMBASE and the Cochrane Library. Search terms included the medical subject headings (MeSH) “noninvasive positive pressure ventilation” OR “noninvasive ventilation” OR “high flow nasal cannula” AND “interstitial lung disease” (Supplemental Table 1). There were no language restrictions.
Study selection and data collection
Two authors (VP and NO) independently performed article selection by title and abstract screening based on predetermined eligibility criteria. The references of the included studies were manually reviewed for additional eligible studies. Disagreements relating to any aspect of the data extraction process were discussed and resolved by a third reviewer (JN), with the final decision made by consensus. The full-text articles of the selected studies were reviewed independently for the final study selection. The data were extracted and analyzed from the included studies (NS, VP, and NJ).
Quality Assessment
Two investigators (VP and NO) assessed the quality of included studies using the Cochrane risk-of-bias tool for randomized trials (RoB 2) and the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) for non-randomized studies tool25,26 (Table 1, Supplemental Tables 2 and 3).
Statistical Analysis
All statistical analyses were performed using Review Manager (RevMan) version 5.4.1 (The Cochrane Collaboration, 2020). We extracted the proportions and 95% confidence intervals (CIs) from each study and pooled them using the random effect model. Cochrane’s Q test was performed and quantified using the I2 statistic to determine the statistical heterogeneity among the included studies. An I2 value of 0% to 25% represents insignificant heterogeneity, greater than 25% but less than or equal to 50% represents low heterogeneity, greater than 50% but less than or equal to 75% represents moderate heterogeneity, and greater than 75% represents high heterogeneity. P-values less than 0.05 were considered statistically significant. A funnel plot visualized the presence of a publication bias (Supplemental Figure 1). The protocol for this study was registered at www.inplasy.com (No. 202260104). Ethical approval was not required.
Results
Search Results
Systematic literature searches identified 1,000 unique citations. A review of titles and abstracts resulted in the elimination of 981 studies. The full text of these 19 studies was reviewed to determine eligibility (Figure 1). This systematic review included ten studies (including a total of 480 patients). One randomized control trial, one prospective cohort study and eight retrospective cohort studies were included.
Study characteristics are described in Table 1. The included studies consisted of NIPPV application (four studies),27–30 HFNC application (two studies)31,32 and NIPPV and HFNC application (four studies).33–36 Idiopathic pulmonary fibrosis (IPF) was a major type of ILDs in this study (239 patients, 49.7% of total ILDs). Three of ten studies included cardiac failure in the definition of acute exacerbation.28,34,36 The ICU was the main location of the interventions (seven of ten studies). The mean duration of noninvasive respiratory supports was 5.76 ± 8.55 days. The mean initial PF ratio before noninvasive respiratory supports was 156.94 ± 64.74.
Publication bias assessment
The funnel plot of the PF ratio outcome of the conventional oxygen therapy and noninvasive respiratory supports was symmetric and showed no publication bias (Supplemental Figure 1).
Effect of intervention
Primary outcome
The PF ratio (a clinical indicator of hypoxemia) is the primary outcome measure of the effect of noninvasive respiratory supports (NIPPV or HFNC) compared to COT on ILD patients with ARF. Of ten studies, pooled analysis was performed on six (NIPPV-four studies, HFNC-two studies)27–32,37 using a random-effect model. This analysis showed that noninvasive respiratory support significantly improved the PF ratio compared to COT. The mean difference was 55.92 (95% CI [18.85–92.99]; I2=88%; p=0.003. In subgroup analysis, both NIPPV and HFNC demonstrated a significant improvement in oxygenation compared to COT (mean difference 51.06, 95%Cl [11.88-90.24]; I2=79%; p=0.01 and (mean difference 67.27, 95% CI [1.17–133.37]; I2=38%; p=0.05), respectively (Figure 2).
Secondary outcomes
Secondary outcomes measures included i.) PaCO2 (a clinical indicator of alveolar ventilation) to examine the effect of noninvasive respiratory supports (NIPPV or HFNC) compared to COT on ILD patients with ARF, ii.) PF ratio to compare the effects of NIPPV with HFNC, iii.) mortality, iv.) intubation rate and v.) hospital length of stay. Four studies (three studies–NIPPV and one study–HFNC)27,28,30,32 reported the effects of noninvasive respiratory supports on PaCO2 outcomes. There was no significant difference in PaCO2 reduction between COT and noninvasive respiratory supports. The mean difference was 3.82 (95% CI [-0.25–7.88]; I2=0%; p=0.07). (Figure 3).
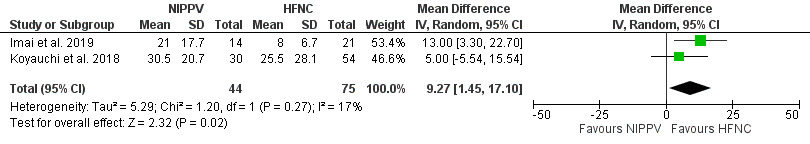
Four studies compared NIPPV directly to HFNC in ILD patients with ARF. Three of four studies33–35 demonstrated a significant increase in PF ratio for NIPPV when compared to HFNC (mean difference 0.45; 95% CI [0.12–0.79]; I2=0%; p=0.008) (Figure 4). The comparison between NIPPV and HFNC revealed that neither method demonstrated a significant impact on mortality (four studies)33–36 (Figure 5) or intubation rates (two studies)35,36 (Figure 6). Risk Ratio (RR) was 1.1; 95% CI [0.83–1.44]; I2=67%; p=0.51 and 1.86; 95% CI [0.42–8.33]; I2=54%; p=0.42, respectively. Lastly, patient groups receiving HFNC experienced significantly shorter hospital lengths of stay when compared to those receiving NIPPV (two studies33,34; the mean difference was 9.27 (95% Cl [1.45–17.1]; I2=17; p=0.02) (Figure 7).
Other effects of NIPPV and HFNC
Two studies conducted on do-not-intubate patients (DNI) examined the effect of oral alimentation and the time of loss of cognitive function before death as outcomes33,34 The HFNC showed a significant oral intake ability before death compared to NIPPV in Imai et al. and Koyauchi et al.; p=0.002 and p=0.037, respectively. In addition, there was significantly less cognitive dysfunction in HFNC compared to NIPPV in Imai et al. and Koyauchi et al.; p=0.03 and p=0.037, respectively.
Koyauchi et al. reported eight adverse events, including seven in patients receiving NIPPV and one in a patient receiving HFNC.34 Specifically, seven of 30 patients (23.3%) receiving NIPPV reported injuries (5 reports of skin damage, one gingival ulcer and one pneumo-mediastinum), while one of 54 patients (1.85%) receiving HFNC reported nasal bleeding. In addition, patients’ requests for interface discontinuation were significantly higher for NIPPV (3 of 30; 10%) compared to HFNC (0 of 54), p=0.043.
In Yogoyama et al.,37 the time to initiate NIPPV determined survival outcomes significantly (p=0.006). The survivor group showed 2.3 ± 2.9 days to initiate NIPPV, whereas the non-survivor group showed 4.4 ± 3.1 days.
Gungor et al.28 demonstrated the APACHE II score greater than 20 and continuous NIPPV demand indicated a significant risk for NIPPV failure: hazard ratio (HR) 2.77 (95% CI 1.19–6.45); p<0.02, and HR 5.12 (95% Cl 1.44–18.19); p<0.01, respectively. On the contrary, the result from Vianello et al.32 conducted in HFNC demonstrated a significantly higher APACHE II score in a success group than a failure group (p=0.043).
Discussion
To our knowledge, this is the first systematic review with meta-analysis to compare the effectiveness of noninvasive respiratory supports (NIPPV and/or HFNC) and conventional oxygen therapy (COT) for improving oxygenation and ventilation in ILD patients with ARF.
Compared with COT, noninvasive respiratory supports significantly increased PF ratio. The subgroup analysis suggested a significant benefit of both NIPPV and HFNC on PF ratio. When comparing NIPPV and HFNC, PF ratio was significantly increased in NIPPV. There was no difference in mortality and intubation rate between the two groups. However, the hospital length of stay showed a significantly shorter duration with HFNC compared to NIPPV. The number of reported serious complications (i.e., pneumothorax, nasal bleeding) was low.
According to ERS recommendation, NIPPV is recommended for ARF with chronic obstructive pulmonary disease exacerbation and/or weaning, cardiogenic pulmonary edema and immunocompromised patients.21 In contrast, HFNC is strongly recommended in acute hypoxemic respiratory failure and conditionally recommended in any high-risk features following extubation or in high-risk and/or obese patients following cardiac or thoracic surgery.20 Notwithstanding, none of the studies explicitly evaluated patients with ILDs.
In our study, noninvasive respiratory supports significantly improved PF ratio compared to COT, and both NIPPV and HFNC subgroups demonstrated this improvement. An earlier systematic review determined that NIPPV improves ventilation-perfusion mismatch and decreases the work of respiratory muscles38 Furthermore, NIPPV has been described as decreasing venous return against pulmonary edema, improving oxygenation, particularly in concomitant cardiac dysfunction patients.39 Similarly, HFNC subgroup analysis demonstrated a significant improvement on PF ratio compared to COT. Prior studies have demonstrated that HFNC improves mucociliary clearance, reducing upper-airway dead space, generating a low level of positive airway pressure with consistent FiO2 regardless of inspiratory flow rate and ultimately providing comfort for the patient.40,41 In our study, PF ratio was significantly increased in NIPPV. This result may be potentially explained by lung recruitment after receiving adequate positive pressure.42,43
Noninvasive respiratory supports did not show a significant reduction in carbon dioxide (CO2) levels. This may reflect many variables (patient’s conditions, various settings of HFNC and NIPPV (CPAP and/or BiPAP), different types of interfaces) across studies. Notably, the risk of carbon dioxide rebreathing was higher on helmet interface.44,45 In a recent meta-analysis of trials of acute hypoxemic respiratory failure, treatment with noninvasive respiratory support strategies was associated with a lower risk of death and intubation46; however, our study demonstrated none of those benefits. Of note, acute pneumonia was a frequent cause of acute hypoxemic respiratory failure in the aforementioned meta-analysis, and the high reversible potential of this acute pneumonia may have contributed to the favourable outcomes described. In our study, HFNC showed a significant decrease in hospital length of stay compared to NIPPV. This finding was consistent with recent studies demonstrating decreased length of stay in patients with hypercapnic respiratory failure and hypoxemia related to COVID pneumonia when using HFNC.47,48 Recent studies have proposed that diaphragm atrophy, a result of positive pressure ventilation, may cause prolonged hospital length of stay in NIPPV patients; however, these positive pressure effects were only related to mechanical ventilation.49,50 Therefore, this issue may require further scientific study.
A strength of this meta-analysis is that it includes a large number of subjects with ILDs with ARF. Our meta-analysis was guided by a registered protocol and strengthened by an extensive search, duplicate citation screening, data abstraction and conducting of prespecified subgroup analyses. However, there are limitations. First, data was obtained primarily from retrospective studies (eight of ten). Thus, the overall level of evidence is low to moderate. Second, summary estimates were limited by heterogeneous types of ILDs, which may interfere with a treatment response and an overall prognosis.
Conclusion
In summary, NIPPV or HFNC might be an alternative modality in ILD patients with ARF, mainly to avoid the negative consequences of intubation. NIPPV showed a significant improvement in PF ratio compared to HFNC. However, there were no mortality and intubation rate benefits when comparing NIPPV and HFNC.
Contributors
NS, VP, and NO conducted the literature searches and resolved discrepancies between citation reviewers, selected studies meeting inclusion criteria, assessed study quality, conducted risk of bias assessment, double-checked data entry, and prepared initial and subsequent drafts of the manuscript and revised versions. NS, NJ and JN conducted literature searches, assisted with screening articles’ study quality, provided methodologic guidance, and integrated comments into the manuscript. All authors revised and approved the final version of the manuscript.
Funding
This study did not receive any funding in any form.
Prior conference presentation
This study was presented as a poster presentation at the 42nd International Symposium on Intensive Care & Emergency Medicine (ISICEM), March 21, 2023.
Competing interests
The authors declare no conflict of interest.
Ethics approval
Not required.
Data availability statement
Data are available on request.