Introduction
Cystic fibrosis (CF) is caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR), an anion channel expressed in epithelial cells throughout the body.1 Due to the malfunction of CFTR (Cystic Fibrosis Transmembrane Conductance Regulator), the balance of sodium (Na+) absorption is disrupted, and the active secretion of chloride ions (Cl-) decreases in the airways.2 This imbalance reduces the volume of airway surface liquid, leading to abnormal mucociliary clearance in the lungs. The resulting mucus buildup promotes bacterial infection and inflammation, ultimately causing lung damage and respiratory failure.3
Emerging evidence suggests that the progression of cystic fibrosis (CF) may be exacerbated by the airway surface’s susceptibility to dehydration.4 Trials examining the potential of hypertonic saline solution (HS) on accelerating mucociliary clearance have recently been proposed as a new option for increasing airway surface liquid hydration in CF patients.5 There is enough evidence to recommend inhaled HS as an alternative mucolytic agent in CF to improve quality of life and reduce pulmonary exacerbations.6 Although most patients tolerate HS treatment well, some adverse events such as coughing, narrowing of the airways, and an unpleasant salty taste have been reported, limiting its use despite pre-treatment with an inhaled bronchodilator.6 Such side effects can lead to poor adherence and decreased rates of inhaled HS use.
In recent years, there has been a growing understanding of how certain components within the lung’s structure play a crucial role in affecting lung damage. Specifically, one of these significant components is hyaluronic acid (HA), a primary material in the matrix that houses cells and fibrous elements like elastin and collagen.7 According to animal studies, HA protects elastin against elastase and regulates neutrophil elastase release. Based on prior research, evidence suggests that HA is protective against various respiratory disorders.8 While a hypothesized function for HA has been shown in experimental animal models of lung emphysema and chronic obstructive pulmonary disease, no data on its therapeutic potential in lung illnesses in people are known.8 Despite the well-known benefits of long-term HS usage on various exacerbations and quality of life, HS safety and tolerability should be improved to avoid poor medication adherence.9,10 Therefore, we sought to investigate the concept that inhaled HS with HA may have beneficial effects on side effects of HS, such as unpleasant taste, cough, throat irritation, and thereby lowering dropout from this treatment (PICO question being P: Patients with cystic fibrosis (CF); I: Hyaluronic acid (HA) administered in conjunction with inhaled hypertonic saline (HS); C: Patients receiving only inhaled hypertonic saline (HS) without hyaluronic acid (HA); O: Cough, Throat irritation, Unpleasant taste, Forced expiratory volume (FEV1) in 1 second).
Methods
This systematic review and meta-analysis have been reported in concordance with guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement (PRISMA).11 Approval from the institutional review board was not required since the data was publicly available.
Search Strategy and Inclusion Criteria
Two reviewers (SEU and MMM) independently searched through the MEDLINE, Embase, and Cochrane central databases from inception until Oct 10, 2022. No time or language restrictions were set. The search strategy involved using MeSH terms to determine the keywords for cystic fibrosis and hypertonic solution coupled with the Boolean operators, ‘AND’ and ‘OR.’ Studies were included if they were (1) randomized controlled trials (RCTs) or observational studies, (2) included patients with a diagnosis of CF, (3) had hypertonic saline with hyaluronic acid as an intervention and (4) hypertonic saline-only as a control group. The PRISMA flowchart in Supplemental Figure S1 summarizes the literature search in detail. We also reviewed other data sources: bibliographies of editorials and relevant reviews from major medical journals, conference proceedings for indexed abstracts, and grey/unpublished literature databases. The detailed search strategy for databases is provided in Supplemental Table S1.
Data Extraction and Quality Assessment
Two reviewers (SEU and MMM) independently conducted the data extraction and verification process using a standardized data extraction form that included trial characteristics and identifiers. Any discrepancies or differences in extracted data were resolved through discussion and consensus between the two reviewers. The original reference articles were reviewed in the event of any discrepancies. Summary events and totals were extracted to calculate risk ratios (RRs) and mean differences (MDs) with 95% confidence intervals (CIs). Other study characteristics were extracted from the total number of participants in each arm, publication year, country of study, percentage of men, length of follow-up, and mean/median ages. The Cochrane Risk of Bias Tool (CRBT) was employed to assess the quality of randomized controlled trials (RCTs) across six domains [selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias].12
Outcome Measures and Statistical Analyses
The outcomes of interest encompassed both objective and subjective measures. Objective assessments included changes in FEV1 measured through spirometry conducted by healthcare professionals following standardized protocols. Subjective assessments were gathered through self-reported measures completed by participants, which involved evaluating symptoms such as cough, throat irritation, and taste perception. These self-reported data were obtained using standardized questionnaires designed to quantify these symptoms and sensations, ensuring a comprehensive evaluation.13 Meta-analysis was performed using RevMan (version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration). Outcomes of interest were presented as RRs with 95% CIs and pooled using a Mantel-Haenszel weighted random-effects model. MDs with 95% CI were pooled using a generic inverse variance-weighted random effects model. The pooled analyses were visually represented with forest plots. Higgins I2 was used to evaluate heterogeneity across studies.13 A 25–49% value was deemed mild, 50–74% moderate, and >75% severe. Publication bias was assessed using Egger’s regression test. A p-value of less than 0.05 was considered significant in all cases.14
Results
Characteristics of Included Studies
After screening 442 publications identified from the initial search, five were included in the final analysis (n=236 patients).15–19 (Supplemental Figure S1) Study characteristics and baseline demographics have been summarized in Table 1. The percentage of males varied from 40.0 to 53.8% between studies. All studies were deemed to be generally low risk of bias according to the CRBT and New Castle Ottawa Scale (Supplemental Figures S2 and S3). Egger’s regression showed no significant publication bias among studies (t=1.57, p=0.12).
Cough
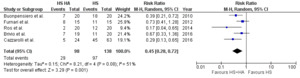
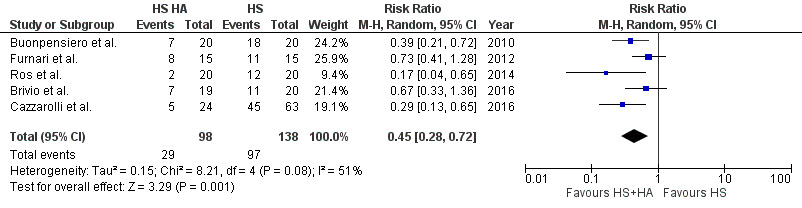
Five studies compared cough in patients taking HS with HA versus those taking HS without HA. A total of 236 patients (138 without HA and 98 with HA) reported data on cough. Compared with patients not taking HA with HS, patients with combined therapy of HS with HA had significantly reduced incidence of cough (RR: 0.45; 95% CI, 0.28 – 0.72, p=0.001) with moderate heterogeneity between studies(I2=51%), (see Figure 1).
Throat Irritation
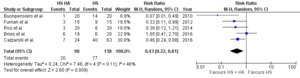
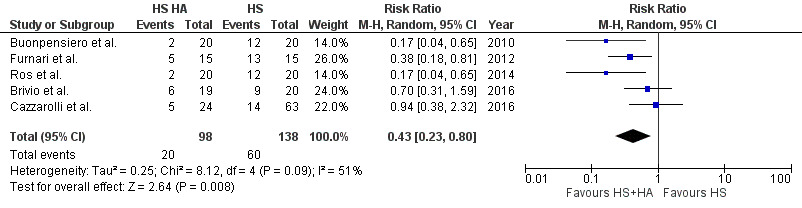
Five studies compared throat irritation in patients taking HS with HA versus those taking HS without HA. A total of 236 patients (138 without HA and 98 with HA) reported data on throat irritation. Compared with patients not taking HA with HS, patients with combined therapy of HS with HA had significantly reduced incidence of throat irritation (RR: 0.43; 95% CI, 0.22–0.81, p=0.009) with mild heterogeneity between studies(I2=46%), (see Figure 2).
Unpleasant Taste
Five studies compared unpleasant taste in patients taking HS with HA versus those taking HS without HA. A total of 236 patients (138 without HA and 98 with HA) reported data on unpleasant taste. Compared with patients not taking HA with HS, patients with combined therapy of HS with HA had significantly reduced incidence of unpleasant taste (RR: 0.43; 95% CI, 0.23–0.80, p=0.008) with moderate heterogeneity between studies (I2=51%), (see Figure 3).
FEV1
Two studies compared FEV1 in patients taking HS with HA versus those taking HS without HA. A total of 63 patients (31 without HA and 32 with HA) reported data on FEV1. Compared with patients not taking HA with HS, patients with combined therapy of HS with HA had significantly reduced FEV1 (MD: -2.97; 95% CI, -3.79—2.15, p<0.001) with no heterogeneity among studies( I2=0%), (see Figure 4).
Discussion
In this meta-analysis of five RCTs, we demonstrated that adding HA to HS significantly reduces cough, throat irritation, and unpleasant taste symptoms and improves FEV1 in patients with cystic fibrosis. These findings are important because when patients experience adverse effects when using inhaled HS alone, there tends to be lower treatment compliance and discontinuation of therapy after a short period.
HS has been observed to produce persistent improvements in mucociliary clearance in individuals with CF, assumed to be related to enhanced airway surface hydration.20 In both short and long-term studies, HS has been proven effective in CF patients, as therapy with HS improves lung function and decreases the number of exacerbations. Moreover, the beneficial effect on the exacerbation rate distinguishes HS as a therapeutically meaningful treatment.21 This may reduce the advancement of lung disease, resulting in enhanced quality of life and a reduction in overall direct and indirect health expenses. However, its clinical utility has been limited due to side effects like cough, throat irritation, and unpleasant taste, leading to frequent HS treatment discontinuation.22 It was proposed that HA combined with HS might be useful in treating CF patients due to its physiochemical features. In a single-dose study, patients expressed a preference for the combination product of HA with HS over HS alone, suggesting potential additive or synergistic benefits.23 In this meta-analysis, we evaluated the tolerability of HS with HA to HS alone in individuals with CF treated with one or the other.24 Our results indicate that adding HA to HS was linked to a better tolerability profile. The addition of HA to HS significantly improved tolerability, leading to sustained reductions in all three outcomes: saltiness, throat irritation, and irritative cough.
Coughing excessively might be an undesirable side effect of hypertonic saline (HS). Coughing is one of the key ways through which HS increases mucociliary clearance and clinical results.25 Given that a considerable decrease in coughing was observed, it is evident that therapy with HA modifies the impact of HS on sputum clearance.
Hyaluronic acid has been demonstrated to have beneficial effects on experimental models of chronic respiratory diseases, inhibiting human neutrophil elastase production, protecting elastin from elastase destruction, preventing tissue kallikrein-mediated bronchoconstriction, enhancing airway epithelial barrier integrity, and stimulating ciliary beating.26 Hyaluronic acid has been demonstrated to have beneficial effects on experimental models of chronic respiratory diseases, inhibiting human neutrophil elastase production, protecting elastin from elastase destruction, preventing tissue kallikrein-mediated bronchoconstriction, enhancing airway epithelial barrier integrity, and stimulating ciliary beating. The intrinsic characteristics of HA are being studied in various fields, including its impact on neuroinflammation, wound healing, and ophthalmology.27,28 Its biological effects are discovered to be molecular weight dependent.26 While low-molecular-weight HA exhibits proangiogenic and proinflammatory action and accumulates after tissue damage and inflammation, high-molecular-weight HA has been demonstrated to protect against macrophage production of cytokines, chemokines, and metalloproteinases.29
In our results, we noted statistically significant advantages of HA in the presence of the examined symptoms and the pleasantness of the inhalation treatment. Based on that, we conclude that HA enhances the use of HS treatment in CF patients. As previously mentioned, it may improve medication tolerance and adherence to frequent inhalations.30 In turn, this may improve related therapeutic outcomes. Our study found no particular causes that might explain the increased tolerance of HS+HA in CF patients. Although HA has been demonstrated to hydrate the airways, attenuate bronchial hyper-responsiveness, and decrease inflammation, the specific mechanism by which HA lessens the negative effects of HS inhalation remains uncertain.31,32 Future studies should investigate precise mechanisms HA exerts in effects when applied in conjunction with HS.
Several factors may have contributed to the heterogeneity observed between the included studies. Variations in study design, such as sample size, inclusion/exclusion criteria, and intervention methods, might have an impact on the results. The use of various patient characteristics and geographic locations can also result in disparate conclusions. Furthermore, variances in research outcomes can be attributed to differences in study timing, potential confounding factors, and changes in medical practices throughout time. Addressing and comprehending these issues correctly is critical for accurately interpreting and synthesizing study data.
Several limitations in this study should be noted. We could not establish the optimum dosage of HS due to the lack of data regarding dosages in the included clinical trials. Also, we could not properly assess the impact of HS on anthropometric measurements due to a lack of data.
Conclusion
In conclusion, this comprehensive meta-analysis underscores the significant benefits of incorporating hyaluronic acid (HA) in the treatment regimen of cystic fibrosis (CF) patients undergoing continuous hypertonic saline (HS) therapy. Our findings highlight that the addition of HA leads to a notably improved tolerability profile, effectively mitigating symptoms such as cough, throat irritation, and unpleasant taste associated with HS. This enhancement in tolerability is crucial for ensuring better adherence to treatment, especially for patients with a history of poor tolerance to HS. By addressing these challenges and promoting greater comfort and acceptability, the combined therapy of HS with HA demonstrates substantial promise in advancing the management and overall well-being of individuals with CF.
Contributors
SEU, MMM and SA contributed to the conception and design of the work. SB, NG, and AC contributed to the analysis and interpretation of data. All authors drafted the manuscript. AN critically revised the manuscript. All authors approved the final version of the manuscript.
Funding
None to declare.
Competing Interests
All authors declare no conflict of interest.
Ethical Approval
Ethical approval was not required because of the use of publicly available data.