Introduction
Trauma-informed care recognizes that trauma, such as abuse and neglect, can have long-term physical and psychological health implications.1 In recent years, Trauma Informed Age Appropriate Care (TiAAC) has emerged as a framework in neonatology prompting a paradigm shift towards decreasing morbidities and premature adult mortality in Neonatal Intensive Care Unit (NICU) survivors.2,3 Tailoring care and providing an environment that is age-appropriate can reduce trauma and potentially improve patient outcomes.4 Trauma can result in toxic stress where the body is under constant duress. NICU hospitalization is a source of trauma for infants who experience separation from their parents, inconsistent caregiving, repeated painful procedures, and an over- and/or under-stimulating environment.5,6 This has been described as Infant Medical Trauma in the NICU.5 Early unmitigated toxic stress can alter neurodevelopmental, physiological and mental health outcomes of NICU survivors.7–9
Five TiAAC core measures described in neonatal literature regarding developmentally-supportive care and age-appropriate practices include; protected sleep, activities of daily living, healing environment; collaborative compassionate relationships, and pain and stress prevention/management.5,10 Each measure represents a set of developmental care practices that acknowledge the needs of the infant and parents while in the NICU.10 The challenge health care providers’ face is facilitating TiAAC best practices for critically ill infants whose survival is often dependent on life saving interventions and technologies.
Use of High Frequency Jet Ventilation (HFJV) to support critically ill infants admitted to Royal Columbian Hospital (New Westminster, British Columbia, Can) 24 bed, level 3 NICU has increased since introduced in July 2013. The LifePulse® HFJV from Bunnell Inc. (Salt Lake City, Utah, USA) ventilates by producing small, gentle, rapid tidal volumes. The LifePulse® uses a Whisper Jet™ patient box (patient box) to produce tidal volumes which are delivered through a proprietary LifePort™ adapter with a built-in jet nozzle connected to the endotracheal tube (ETT). Though beneficial as a gentler mode of ventilation, the limited length of the HFJV circuit tubing, between the pinch valve tubing and LifePort™ adapter introduced two key challenges related to the provision of TiAAC.5
First, due to the length of tubing, the patient box, a constant source of adverse auditory and vibratory stimulation, had to be placed inside the incubator next to the infant’s head, potentially impacting infant’s sleep and undermining a healing environment. Assessing, supporting, and protecting well-organized sleep is associated with decreased stress, improved developmental outcomes, growth and autonomic stability.5 A healing environment including appropriate tactile, vestibular, olfactory, auditory and visual sensory exposure supports rest, healing and recovery.5 A TiAAC best practice to protect sleep and provide a healing environment is limiting exposure to excessive sounds, which exceeds the preterm infant’s capacity to cope.5,11 Recommendations suggest that noise levels above 45 to 50 dB should be avoided,12–14 though keeping noise below 45 dB in the NICU is rarely achieved.12–16
The sound generated by the patient box is between 52.4 and 55.3 dB depending on the settings and model.13 When factoring in other sounds inherent to the NICU environment in conjunction with the patient box, the continuous noise levels infants are exposed to exceed recommendations and may surpass the infant’s ability to cope, creating a suboptimal environment for neuroprotection and neurodevelopmental outcomes.
The second challenge is that infants were not held skin-to-skin (S2S). The length of the circuit tubing made it challenging to support S2S therapy safely. S2S therapy addresses the traumatic aspects of a NICU admission, including maternal separation, sleep deprivation, sensory isolation, unmanaged/undermanaged pain, and dehumanization.4,5 It is a core TiAAC intervention for neuroprotection and mitigation of toxic stress.17 Evidence concerning S2S therapy on respiratory devices, specifically High Frequency Oscillator Ventilation (HFOV) and HFJV is limited. Studies of stability during S2S therapy have shown variable results and have been mostly on stable non-intubated infants.18 Small observational studies have shown varying results.18–21
Criteria and procedures for providing safe S2S therapy have been published20 and shared on social media groups and inter-hospital discussion forums. Strategies and criteria vary widely, with valid concerns about accidental unplanned extubation, deteriorating vital signs, and other adverse events to both infants and parents.19 With these concerns in mind and applying a TiAAC lens, we aimed to improve the NICU experience for infants on HFJV and their parents to minimize the stress and trauma of their NICU hospitalization by decreasing exposure to noise and facilitating S2S therapy.
Methods
Context
This quality improvement (QI) project was conducted in a 24-bed tertiary center NICU. As one of four tertiary-care NICU referral centers in the province, this unit cares for in-born and out-born preterm and critically-ill infants requiring complex medical care. Surgical cases are transferred to the provincial quaternary center. There are approximately 3000 births per year at Royal Columbian Hospital. The NICU admits over 450 infants annually; approximately 20% of admissions are less than 32 weeks gestational age at birth and 10% are less than 29 weeks, with nearly 75% of admissions being in-born.
Interventions
This quality improvement project was based on three key drivers: the patient box should be placed outside of the incubator, safe S2S therapy should be provided while on HFJV, and a positive experience should be provided to both the infant and their parents. Four main interventions were undertaken by the team.22
Intervention 1- Move patient box outside incubator
To move the patient box outside of the incubator, the tubing between the patient box and ETT had to be extended and a functional external shelf had to be designed and built. Bench testing of the tubing extension conducted assessed the effect of adding an extension between the patient box and the ETT on ventilator function, including measured Peak Inspiratory Pressure (PIP), measured Positive End-Expiratory Pressure (PEEP), Mean Airway Pressure (MAP) and Servo. Internal bench testing of a 13 cm extension (11 cm of tubing and 2 cm connector) demonstrated no effect on ventilator function (see Figure 1). There was no benefit to extending the tubing beyond 13 cm because of the length of the LifePort™ Adapter and bench testing showed ventilator function was impacted (see Figure 1). External bench testing by Bunnell Inc. replicated these findings. Though Bunnell Inc. has not extended the length of the manufacture tubing based on FDA approval, they are aware of the modifications we have made.
Securing the patient box outside of the incubator required the team to design and build a shelf that was stable, had brakes, could swivel and move up and down. In collaboration with the biomedical engineer department, the clinical team repurposed existing equipment to meet this need.
Intervention 2 - Implement regional S2S therapy guidelines and develop local HFJV eligibility criteria
In October 2016, the health region rolled out a S2S therapy clinical practice guideline that included stability-based S2S therapy eligibility criteria and recommendations to use the standing transfer method for all infants. Applying the stability-based approach, the team drafted criteria for S2S therapy on HFJV and then reviewed, modified and approved through feedback cycles. Stakeholders who consulted in the feedback cycles included parents, Neonatologists, Respiratory Therapists (RTs), Occupational Therapists, Physiotherapists, Social Workers, Lactation Consultants, Management and Nurses. Feedback was elicited at meetings, through one on one conversations, and simulations of the transfer process. To be eligible for S2S therapy on HFJV the infant had to meet the following criteria:
-
ETT securement confirmed
-
on HFJV for greater than 48 hours
-
stable fraction of inspired oxygen (FiO2) and MAP requirements
-
stable gases and transcutaneous carbon dioxide (TcCO2) for the past 12 hours
-
no requirement for positive pressure ventilation (PPV) for greater than 30 seconds in the past 24 hours
-
no resuscitation requiring neonatologist intervention in the past 24 hours
-
tolerates short, intermittent disconnection
-
12 hours post surfactant administration
-
hemodynamically stable with no acute concerns
Stability and tolerance related criteria above was determined on a case by case basis by the multidisciplinary team within the context of the individual infant’s condition. The criteria has remained the same since implementation.
Intervention 3 – Develop standing transfer process and parent/staff education
If not done in a coordinated and systematic way, transferring the infant to and from S2S therapy, even using the standing transfer method, could result in an accidental unplanned extubation and physiologic de-stabilization. Therefore, a standardized transfer process and checklist were developed outlining steps and considerations in the provision of S2S therapy prior, during, and after transfer of the infant from their bed to their parent. The checklist can be found in Supplement 1. The checklist was co-developed with a mother in the NICU, whose baby was on HFJV. Together with the mother, the team trialled and adapted the process and checklist through a series of simulated transfers using different scenarios (e.g. decompensation, disconnection) using a teddy bear, prior to the first infant transfer. Role clarity and intentional, clear communication was essential to safe transfers. Simulations with return demonstration prior to the first infant transfer was integrated into the standardized education process for all families and staff.
Infants on HFJV are nursed 1 to 1 in the unit with RT caseloads of 1 to 3 and up to 1 to 5 depending on acuity. There are two designated RTs in the NICU, therefore S2S therapy is dependant on staffing levels. The transfer process requires four trained staff to assist the parent, including two RTs. Initially, S2S therapy on the HFJV only occurred during day shifts, when trained staff were available to support the team and staffing levels were sufficient to accommodate the transfer process.
Intervention 4 – Improve equipment and expand education
With funding support from innovation grants and in collaboration with the biomedical engineering department, the bedside shelf for the patient box was improved and is now an arm known as the “ReaCH” with the slogan “Ready, Set, Jet”. The ReaCH arm attaches to the HFJV stand, decreasing the floor space required at the bedside and allows for more versatile positioning and reliable securement of the patient box. Every HFJV is now fitted with a ReaCH arm.
As of January 2017, S2S therapy is a standard of care offered to all infants on HFJV based on local eligibility criteria. Staff are oriented to the HFJV S2S transfer process and checklist during orientation to the care for infants requiring HFJV. In addition to simulated transfers, family education now includes a pamphlet (co-created with parents), highlighting 'what past parents want you to know" with a step-by-step transfer video recorded with parent partner and their infant.
Measures and Analysis
A mixed-methods design was used, including retrospective chart reviews (quantitative) and semi-structured interviews (qualitative).
Quantitative Data: Safety
Outcome measures to evaluate the safety of S2S therapy while on HFJV in the NICU were collected through chart reviews. Infants were identified by reviewing admission/statistic logs and care plans. A chart review was conducted if the infant was admitted to the NICU between January 2017 and December 2018 and received respiratory support on HFJV. Data collection started in January 2017, as S2S therapy for infants on HFJV was considered a standard of care at this time with improvements to existing education, processes and equipment occurring after that.
Charts were reviewed retrospectively for demographic and descriptive data, as well as primary and secondary outcome measures. The primary outcome measure assessing safety of S2S therapy was unplanned extubation (premature removal of the ETT by the infant, during care, or blocked ETT)23 during S2S therapy. The secondary measures, assessing physiological stability, were assessed by reviewing documentation:
-
apnea, bradycardia and/or desaturation during S2S therapy
-
manual breaths via the ventilator or manual PPV during S2S therapy
-
HFJV changes in the 12 hours post S2S therapy
-
respiratory indicators in the 12 hours pre compared to the 12 hours post S2S therapy, including:
-
hourly TcCO2
-
hourly FiO2
-
apnea, bradycardia and/or desaturation
-
Apnea was defined as a pause in breathing > 20 seconds, or > 10 seconds if accompanied by a bradycardia or desaturation); bradycardia as <100 beats per minute for infants ≤ or equal to 37 weeks post menstrual age (PMA), or < 80 beats per minute if greater than 37 weeks PMA; and desaturations as oxygen saturation < 80%.24
Data were analyzed using descriptive statistics, frequencies and proportions as appropriate. Pair-wise comparisons of secondary outcome measures were completed using dependent t-test. All analyses were completed using 2-tailed tests and alpha <0.05.
Qualitative Data: Experience
Semi-structured interviews were conducted after all the interventions were complete with parents and staff to further understand the perceived impact, benefit, and persistent barriers of S2S therapy while on HFJV. Positioning of the patient box was not a focus of the interviews. A convenience sample was used; staff and parents were recruited in person and through email. Participation was voluntary and written informed consent was obtained. Interviews were conducted in person, over the phone, or in writing at the participants’ convenience, by one of the authors (D.V., S.G. and S.R.). An interview guide with open-ended questions was used for all interviews. Verbal interviews were audio recorded and transcribed verbatim, interview questions can be found in the supplementary information files.
The six-step process described in Braun and Clark25 was used to complete an inductive thematic analysis of the parent and staff interviews. The steps included data familiarization, generating initial codes, searching, reviewing, defining and naming themes, and producing the report. All four authors independently reviewed all interview transcripts to become familiar with the data and generated initial codes. Following initial coding through an iterative process, the authors met in person to search for, review, define and name themes.
Ethical Considerations
The hospital’s Research Ethics Board deemed this study a QI project and exempt from ethics approval. A data access agreement was approved by the hospital’s Privacy Department to conduct retrospective chart reviews and the written consents (staff and parents and interview tools) were endorsed.
Results
Quantitative Data: Safety
Thirty-two infants were identified as receiving respiratory support on HFJV between January 2017 and December 2018. Of the 32 infants, 13 were excluded from the chart review (one infant was not on HFJV and 12 infants were not held for S2S therapy while on HFJV). The majority of infants on HFJV who were not held (n=11) did not meet the unit’s stability criteria for S2S therapy, the remaining infant was not held due to parents’ comfort level. A total of 19 infants were included in the chart review.
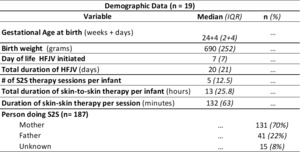
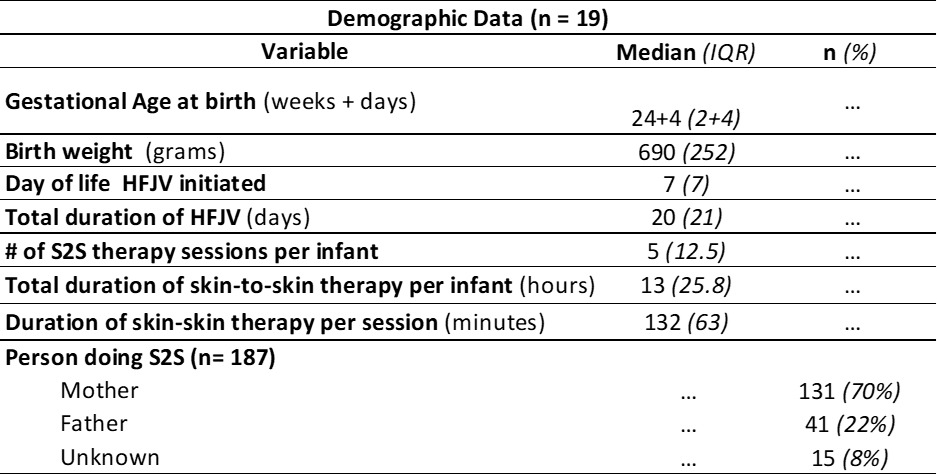
Demographic data for the study sample (n=19) is shown in Table 1. There were 187 S2S therapy sessions documented, totalling 407 hours for the 19 infants. The median and the interquartile range (IQR) of S2S therapy sessions per infant was 5 (12.5), with each infant having a median (IQR) of 13 hours (25.8) of S2S therapy total. The mean (IQR) per session was 132 minutes (63). Seventeen infants (184 S2S therapy sessions) were included in analysis of primary and secondary outcome measures; two infants were palliative and extubated during the S2S therapy session.
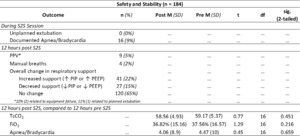
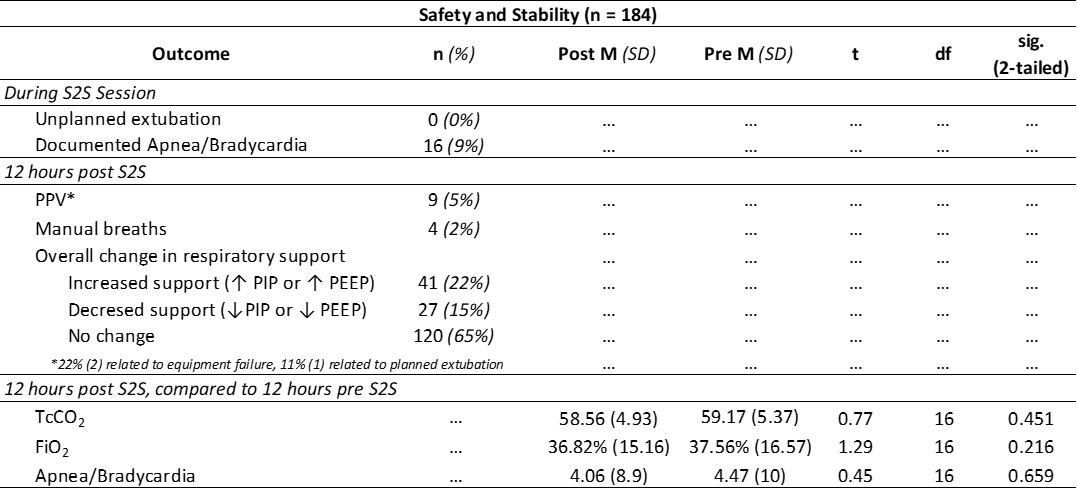
Outcome data is shown in Table 2. During the study period, there were no documented unplanned extubations during S2S therapy. During 9% (n=16) of S2S therapy sessions, there was at least one documented apnea, bradycardia and/or desaturations.
In the 12 hours post S2S therapy, manual breaths via the ventilator were required 2% (n=4) of the time, while PPV was required following 5% (n=9) of S2S therapy sessions. In the 12 hours following S2S therapy, ventilation support was weaned or maintained 80% (n=147) of the time.
Respiratory indicators for the 12 hours prior to S2S therapy sessions were compared to the 12 hours post S2S therapy; there was no significant difference in any of the variables. There was no significant difference in TcCO2 in the 12 hours prior to S2S therapy (M=59.17, SD=5.37) and the 12 hours post (M=58.56, SD=4.93), t (16) =0.77, p=0.45. TcC02 either decreased or remained the same 50% (n=87) of the time when comparing all available pre and post means (n=175).
Likewise there was no significant difference in FiO2 in the 12 hours prior to S2S therapy (M=37.56%, SD=16.57) compared to the 12 hours post (M=36.82%, SD=15.16), t (16) =1.29, p=0.22. Mean FiO2 requirements either decreased or remained the same following 47% (71) of the 151 S2S therapy sessions (infants on 100% Fi02 at the time of S2S therapy were excluded from this analysis due to limitations in assessing stability based on FiO2 adjustments). 60% (39/65) of the time, the number of documented apnea, bradycardia or desaturations decreased or remained the same following S2S therapy when compared to the 12 hours pre S2S therapy. There was not a significant difference found between the mean documented number of apneas, bradycardias or desaturations in the 12 hours prior to S2S therapy (M=4.47, SD=10) compared to the 12 hours post (M= 4.06, SD=8.9), t(16)=0.45, p=0.66.
Qualitative Data: Experience
Six parents participated in five interviews, including four mothers and two fathers (one interview included both the mother and father). Of the five interviews, two interviews were in person, two over the phone, and one in writing. Verbal interviews were an average of 8:23 minutes long (range 3:49 to 12:14 minutes). Eleven staff interviews were conducted, including six Registered Nurses, two RTs, two Neonatologists and one Occupational Therapist. All interviews were in person and were an average of 6:33 minutes in length (range 2:55 to 10:46 minutes). Thematic analysis of parents and staff interviews revealed the following themes: Strengthening bonds; Be brave, Just breathe; I see so much good; Small steps for tiny people; It takes a village; It changes everything. Figure 2 outlines the identified themes and the components along with exemplary quotes; a full summary is available in Supplement 1.
Discussion
This QI project focusing on mitigating trauma for infants and their parents by enabling S2S therapy while on HFJV, demonstrated safety and was perceived by parents and staff as beneficial. Despite initial parental fears about S2S therapy, parents described their experience as improving infant-parent bonding and their ability to cope. Staff and parents perceived S2S therapy while on HFJV as beneficial to the infant and in some cases maternal milk production and parental-staff rapport building. Both parents and staff recognized the importance of a standardized transfer process, teamwork and clear communication to the safety of S2S therapy while on HFJV. In the NICU, S2S therapy on HFJV is now the standard of care due to these results, with no unplanned extubations or patient safety events reported since implementation of this QI project in 2017. The S2S therapy criteria for infants on HFJV and the transfer process remain the same. There are now more staff trained to aid in the transfer for S2S therapy, which allows more than one infant to receive S2S therapy at a time, and to support S2S therapy 24 hours a day.
These results are consistent with prior publications demonstrating safety and the benefits of S2S therapy for preterm and critically ill infants in the NICU as a TiAAC intervention.4–6,10,17–21 However, this study is the first, to our knowledge, looking at S2S therapy for infants on HFJV.
This study has several strengths. First, it demonstrates the ability of a committed multidisciplinary care team to institute seemingly small but meaningful change to improve care through a TiAAC lens at the local level using QI methodologies. In doing so, it highlights the importance of acknowledging and addressing safety concerns as well as parental, infant and staff experiences. Second, the study demonstrates for the first time, to our knowledge, that extending the HFJV circuit to position the patient box outside is feasible to support a healing environment and protected sleep as well as facilitate S2S therapy. A third benefit is that it validates S2S therapy can be provided to infants on HFJV with perceived benefits similar to those in other NICU populations. Finally, it confirms the importance of teamwork, clear communication, as well as standardized processes and education in safe and successful implementation of practice changes.
This QI project is limited in regards to the post implementation design, retrospective nature of the chart reviews, reliance on clinical documentation and small number of eligible infants. Results should be interpreted and applied with caution due to the small sample size. Larger QI projects/research would be beneficial to support this as a standard of care. It would have been beneficial to conduct pre and post implementation chart reviews for comparison of outcome measures. The small sample size of interviewees and potential selection bias from use of a convenience sample are also limitations. Finally, Bunnell Inc. has not altered their circuit tubing, which may be a barrier to broad implementation of this practice.
We are hopeful that other units will be encouraged to look at their current set-up and care practices for infants on HFJV. With S2S therapy, a foundational intervention for supporting TiAAC core measures, they can learn from and build upon the team’s work to implement local solutions to bring infants on HFJV and their parents safely together. The team has created an information guide and worked with biomedical engineers to improve the design of the external shelf to hold the patient box (see Figure 3).
Acknowledgments
We would like to thank the FHA Innovation Grant Committee and the Royal Columbian Hospital Foundation for sharing our vision and supporting our project. We are grateful to Brittani Barber, Courtney Nickerson and KC Solano for conducting the chart reviews. We would like to acknowledge the hard work of the RCH NICU team (including, but not limited to Neonatologists, Nurses, Respiratory Therapists, Occupational Therapists, and Physiotherapists) who have worked collaboratively to bring infants on HFJV together with their parents. Thank you to the Biomedical Engineering teams that were innovative and created the equipment that is now known as the “ReaCH Arm,” which was critical to the success of this work. Thank you to Bunnell Inc. for creating and supporting a life saving therapy that has allowed infants to survive and go home with their families. Thank you to the Fraser Health Department of Evaluation and Research for your support in this endeavor. Finally, we are indebted to Ashley Durance, whose passion, bravery and persistence as a mother nurtured this project as if it was her own.
Contributors
All authors contributed to the conception or design of the work, the acquisition, analysis, or interpretation of the data. All authors were involved in drafting and commenting on the paper and have approved the final version.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Ethical approval
The hospital’s Research Ethics Board deemed this study a QI project and exempt from ethics approval.
Data Availability
The dataset generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AI statement
The authors confirm that no generative AI or AI-assisted technology was used to generate content.