Introduction
In March 2020, the World Health Organization (WHO) declared COVID-19 a pandemic. Despite public health interventions, COVID-19 spread persistently, and about 615 million cases were diagnosed with more than six million deaths.1 COVID-19’s symptoms vary widely across patients and range from mild (i.e., fever, fatigue, headache, and cough) to severe (i.e., difficulty breathing, severe illness, hospitalization, and death).2 Even after fully recovering from the disease, many studies reported acute post-COVID-19 symptoms such as fatigue syndrome, cognitive impairment, dyspnea, anosmia and ageusia.3–5
At the beginning of the pandemic, pharmacological approaches for the management of the symptoms of COVID-19 were not defined. Several existing medications were tried (e.g., Ivermectin, Remdesivir, hydroxychloroquine) but were not proven beneficial in supporting COVID-19 patients.6–8 In contrast, other medications (e.g., monoclonal antibodies) were shown to reduce the duration of hospital length of stay (LOS) and mechanical ventilation.9,10 Finally, systemic corticosteroid therapies were associated with reduced mortality rates in hospitalized patients.11,12 However, systemic therapy was not recommended for noncritically ill patients13 due to potential immunosuppression risks and systemic side effects.
Inhaled corticosteroids (ICS) and intranasal corticosteroids (INC) were also reported to be beneficial in the treatment of COVID-19 patients. Several theories have been proposed to explain their beneficial effects, including, but not limited to, a reduction in the expression of proteins responsible for the virus’s entry into the host cell,14 a downregulation of coronavirus genes,15 inflammatory modularity actions and improved t-cell response and reduced epithelial cell damage.16
Current evidence about ICS effectiveness in COVID-19 patients is mixed.17–19 A study by Ezer et al. concluded that ICS and INC did not benefit COVID-19 patients.18 Another study by Ramakrishnan et al. reported that the early use of ICS and INC reduced the time to recovery and medical care needs.17 Therefore, INC’s efficacy and safety are debatable, and more reliable data are required to clarify the risk-benefit ratio.
This review seeks to synthesize studies evaluating the effectiveness of inhaled corticosteroids (ICS) and intranasal corticosteroids (INC) on mortality, hospital length-of-stay (LOS), and improvement of smell scores in patients with COVID-19 (PICO question being P: COVID-19 patients; I: Inhaled corticosteroids or Intranasal Corticosteroids; C: Placebo; O: Mortality, Length of hospital stay, Improvement of smell).
Methods
The study was designed according to the Cochrane Handbook for Systematic Reviews of Interventions and reported under the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.20,21
Systematic literature search
A search was conducted in the following databases: Embase, Web of Science, PubMed, Cochrane Library, and Scopus. The search was conducted from inception until August 27, 2022, using the following terms (COVID OR COVID19 OR “SARS-CoV-2” OR “SARS-CoV2” OR SARSCoV2 OR “SARSCoV-2” OR “SARS coronavirus 2”) AND (“inhaled corticosteroids” OR ICS). The complete search strategy is provided (Supplementary Table 1).
Eligibility criteria and study selection process
We included randomized controlled trials (RCTs) that met the eligibility criteria: 1) Population: patients infected with COVID-19; 2) Intervention: treated with ICS or INC; 3) Comparator: control; 4) treatment Outcomes: mortality, hospital LOS, improved sense of smell, alleviation of COVID-19 related symptoms, time to self-reported recovery. Studies that did not report on at least one of the above treatment outcomes were excluded. The titles and abstracts of identified studies were reviewed for eligibility, and those meeting the criteria were advanced to full-text review. The systematic literature search was conducted by two authors independently, and conflicts were resolved by including a third author. References of the included trials were reviewed to identify additional relevant studies. Two authors defined relevant study characteristics and then, working independently, extracted data. A third author validated the outcomes of the extraction process.
Quality assessment
Included studies were evaluated for bias using the Cochrane Risk of Bias tool for RCTs (version 1).21 This tool prompts critical assessment in the following domains 1) randomization of the population; 2) allocation of arms; 3) participant and investigator blinding; 4) assessment of outcomes and their blinding; 5) detection bias and other biases. Judgment can be a high, low, or unclear risk of bias. The GRADE approach was used to rate the certainty of evidence across included studies.22
Data extraction
We extracted 1) summary data and baseline characteristics of the study’s populations, including Study ID, study arms and the route of corticosteroid administration, study site, age, follow-up, comorbidities, outcome measurement tools, inclusion criteria; 2) outcomes data: mortality, LOS, visual analog scale (VAS) of smell score, participants with alleviation of COVID-19–related symptoms, and time to self-reported recovery.
Data synthesis
Data were analyzed using Review Manager (RevMan) software version 5.4. In the case of dichotomous data, we presented data as risk ratios and 95% confidence intervals (95% CI). Mean differences and 95% CI were used with continuous s data. Heterogeneity was tested using the chi-square test and I-square test (I2). A significant difference was reported if the p-value < 0.05. Data were considered heterogenous if the p-value of chi-square < 0.1 and the I2 value was greater than 50%. We pooled data in the random effects model if it were heterogeneous, while the fixed effect model was used for homogenous data. We performed a subgroup analysis based on the weeks the data was reported. We used the Cochrane leave-one-out technique when we could not resolve the heterogeneity by leaving one study out from the analysis.21
RESULTS
Literature search and study selection
Based on our research strategy, we retrieved 2221 unique articles. After title and abstract screening, 23 studies were suitable for full-text screening. Ten studies met our inclusion requirements. The 13 excluded studies were non-RCT study designs and failed to include the defined outcome measures (see Figure 1).
Study characteristics
The ten included studies had a combined 3168 patients with a mean age of 44. Two studies were conducted in the United Kingdom, two in Iran, and one each from Egypt, Syria, the United States, France, Canada, and Korea. Included studies had follow-up duration between one to four weeks. Characteristics and outcomes of included studies are presented in Table 1.
Quality of the included studies
Our included RCTs had a low to moderate quality regarding the risk of bias assessment and the specific items and details in Figure 2. The GRADE assessment revealed very low to moderate overall evidence quality (see Supplementary Table 2).
Outcomes
Mortality
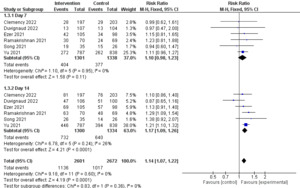
Five studies included mortality as an outcome measure,18,19,23–25 with a total sample of 2,414 patients. There was no significant difference in mortality between the ICS and placebo arms (RR= 0.69, 95% CI [0.36 to 1.35], p-value = 0.28). These pooled studies were homogeneous (p-value = 0.45, I2 = 0%). See Figure 3.
Length of stay
Three studies reported data on LOS.23,25,26 Pooled analysis of a sample of 261 patients showed a significant difference. Patients receiving ICS had shorter LOS when compared to those receiving the placebo (MD = -1.52, 95% CI [-2.77 to -0.28], p-value = 0.02). We found homogeneity with a fixed model between the included studies (p-value = 0.71, I2 = 0%). See Figure 4.
Intranasal corticosteroid and improvement in sense of smell
Three studies examined the effects of INC on patients’ recovery of the sense of smell, measured across three different time points after the initiation of INC.27–29
Week 1: Two studies27,28 reported this outcome in week 1 with a total sample of 177. Our analysis revealed no significant difference between the INC group and placebo, with (MD = 0.32, 95% CI [-1.21 to 1.84], and p-value = 0.68). The results were heterogeneous in the random model (p-value = 0.10, I2 = 64%). The heterogeneity was not resolved even after the random effect model (see Figure 5).
Week 2: When evaluated at week 2, the pooled results were insignificant across study arms,27–29 with an (MD = 0.23, 95% CI [-1.32 to 1.79], and p-value = 0.77). The population of studies was 247. The heterogeneity could not be removed with the random model or the leave-one-out technique (p-value = 0.06 I2 = 65%). See Figure 5.
Weeks 3-4: The studies used in the analysis27–29 showed no significant difference between the INC group and placebo, with an (MD = 0.69, 95% CI [-0.86 to 2.23], and p-value = 0.38). The heterogeneity was observed ( p-value = 0.03 I2 = 71%). See Figure 5.
After we performed the leave-one-out technique, the heterogeneity was resolved by removing Kasiri et al. with a p-value = 0.83 and I2 = 0%.28 Our results became significant with a sample size of 170, favouring the INC patients over the placebo (MD = 1.52, 95% CI [0.27 to 2.78], p-value = 0.02). See Supplementary Figure 1.
Alleviation of post-acute COVID-19 syndrome symptoms
Six studies included data about the effect of ICS on post-acute COVID-19 syndrome symptoms (body temperature ≤ 37.5C and reports of all following symptoms as minor or none, with no subsequent relapse: asthenia, headache, cough, retrosternal discomfort/pain, thoracic oppression, thoracic pain, dyspnea, nausea, vomiting, diarrhea, abdominal pain, anorexia, myalgia, or arthralgia).17–19,24–26 Subgrouping analysis was applied depending on the days of alleviation of symptoms on days seven and 14. Data on day seven were insignificant, with a (RR = 1.10, 95% CI [0.98 to 1.23], and p-value = 0.11). The pooled data on day seven were homogenous (p-value = 0.95, I2 = 0%). While on day 14, our results were significant in favour of the ICS group over the placebo (RR = 1.17, 95% CI [1.09 to 1.26], p-value < 0.0001). All studies in this subgroup were also homogenous (p-value = 0.24, I2 = 26%). In the comprehensive analysis, the studies were homogenous (p-value = 0.60, I2 = 0%). See Figure 6.
Time to self-reported recovery
Only two studies were suitable for this estimate, with a collective sample size of 307. Results revealed insignificant differences (MD = -1.28, 95% CI [-6.77 to 4.20], p-value = 0.65). The populations from the studies were heterogenous (p-value = 0.0003, I2 = 89%), and the random model could not resolve the heterogeneity (see Supplementary Figure 2).
Discussion
This study demonstrates significant reductions in LOS and improvements in select post-acute COVID-19 symptoms in patients randomized to ICS treatment. On the other hand, there is no significant difference between the ICS and placebo groups in mortality and time to self-reported recovery measures.
After adjusting for heterogeneity, our results showed a significant increase in VAS smell score in the INC groups when measured in the third to fourth week. Anosmia is one of the most prevalent post-COVID symptoms.25,30,31 The prevalence of smell problems among COVID-19 patients was 53.5%.32 While most anosmia patients recover after a few months, a small percentage appears to have a persistent smelling malfunction with severe clinical manifestations such as phantosmia or parosmia. Moreover, the mechanism of anosmia after COVID-19 infection is still unclear.33 Olfactory cleft syndrome, early apoptosis of olfactory cells, modifications to olfactory cilia and odour transmission, an impact on olfactory bulbs, an injury to epithelial olfactory cells, damage to olfactory neurons, or issues with stem cells have all been suggested as possible causes.30 Several treatments have been examined for their effectiveness in treating this and other post-acute COVID-19 symptoms. A longitudinal study found that palmitoylethanolamide, luteolin treatment, and olfactory training may improve memory and olfactory dysfunction.34 Theophylline was also tested for nasal irrigation; the results were inconclusive.35 Omega-3 is suggested for the treatment as well.36
The literature reported using oral corticosteroids to treat olfactory dysfunction.37,38 Nevertheless, using nasal corticosteroids with post-covid anosmia is questionable, and many experts still recommend it. For instance, Kasiri et al. concluded that combining ICS with olfactory training could improve olfactory dysfunction more than olfactory training alone.28 We removed Kasiri et al.28 from the analysis by the “leave one out” method to resolve the heterogeneity because it had different results from the other studies. So, we need to do more studies that measure this outcome. Additionally, Vaira et al. reported that a combination of oral corticosteroids with inhaled ones reduces long-term anosmia.39 Furthermore, Singh et al. concluded that using INC with triamcinolone oral paste significantly improved the olfactory and test function.40 At seven and 14 days, our three studies27–29 found no statistically significant difference between INC patients and placebo in terms of scent improvement. In contrast, INC patients considerably outperformed the placebo group in weeks three and four. Our meta-analysis demonstrated that the INC may increase the VAS smell score, even though the individual studies’ findings were minor.
Rashid et al. mentioned that 83% of anosmia patients recovered after 30 days.41 Therefore, our observations may be attributed to the time passing, not the effect of INC. Additionally, our mean age in the studies of INC patients was 32.11, with an SD of 12.6. The mean age in the control group was 31.6, with an SD of 11. Ruth et al. reported a significant difference in smell improvement between patients below 40 years old and above 40.42 Therefore, age also was a strong confounder in our results. Our sample size was relatively small, and many confounders can affect the results. Therefore, more studies with different treatment periods and patients of different ages are needed to elucidate this outcome and evaluate the available treatments for this problem. Nevertheless, INC may positively impact patients’ recovery from anosmia after receiving them for three to four weeks, and olfactory training was the standard solution with or without medications.
Our results confirmed that the alleviation of post-acute COVID-19 symptoms (body temperature ≤ 37.5C and reports of all following symptoms as minor or none, with no subsequent relapse: asthenia, headache, cough, retrosternal discomfort/pain, thoracic oppression, thoracic pain, dyspnea, nausea, vomiting, diarrhea, abdominal pain, anorexia, myalgia, or arthralgia) was significantly higher in patients receiving ICS, after two weeks of treatment. These results are compatible with the previous meta-analysis by Chen et al.43 Furthermore, our sample size was larger than Chen et al.43
ICS did not improve the mortality rate of hospitalized COVID-19 patients. Five studies in the meta-analysis found no significant difference in mortality between the ICS and placebo groups.18,19,23–25 Duvignaud et al. discussed that it was difficult to conclude that ICS may improve the symptoms of COVID-19 patients or decrease the mortality rate even in a larger population.24 These results are compatible with the conclusion of the previous reviews, which suggested that corticosteroids could not reduce the risk of hospitalization or mortality in COVID-19 patients.44,45 Moreover, a retrospective study from Spain found that inhaled corticosteroids did not decrease the mortality rate in COVID-19 patients.46 To conclude, on the evidence available, ICS treatment does not reduce the mortality rates in COVID-19 patients.
This meta-analysis demonstrated that ICS significantly reduced LOS. However, we should consider these results cautiously because they depend on three studies, two of which reported non-significant results. Two studies25,26 showed no significant difference in LOS, while a third concluded that the ICS reduced the LOS.23 These discrepancies may be attributed to each study’s inclusion and exclusion criteria. For example, Song et al.26 only included mild to moderate patients. Also, they excluded patients with less than 95% oxygen saturation, immunocompromised patients, or patients with comorbidities. Furthermore, even though Yu et al. included patients with comorbidities and weakened immune systems, their trial was conducted remotely in primary care centers.25 Nevertheless, Alsultan’s sample was isolated in hospitalized patients with less than 93% oxygen saturation plus other symptoms such as a respiratory rate of less than 30 per min or a CT scan with infiltrate > 50%.23 Therefore, it is necessary to conduct further research on this topic. As we have seen in the pandemic, reducing the length of stay (LOS) in the hospital could also be a lifesaving measure for many other patients. Inhaled corticosteroids (ICS) may have the potential to decrease the duration of hospitalization by lowering the inflammation in the body.16 In vitro research has demonstrated that ICS has antiviral effects through the downregulation of ACE2 and TMPRSS2 gene expression, which have an essential role in the virus’s entrance to the cell47 and decreased SARS-CoV-2 replication in epithelial cells of the airway.48 Our study collected the evidence and concluded that ICS might significantly lower LOSs.
Two studies examined the effects of ICS on time to recovery from COVID-19 symptoms.17,23 Ramakrishnan et al. reported a significant difference between the ICS and placebo groups.17 At the same time, the meta-analysis showed no significant difference between the ICS group and the placebo. However, this outcome is very subjective, and studies could not rely on it alone in the investigation. As a result, studies with larger populations are needed.
Limitations
The studies included in our meta-analysis were at moderate risk of bias, so higher-quality RCTs are warranted. We are the first meta-analysis to investigate the VAS smell score, mortality, and LOS outcomes. However, several limitations can affect our analysis. First, pooling patients of different health statuses may affect the data’s validity. Including six unblinded RCTs may also affect the results by reporting bias.17,23–27 Secondly, the heterogeneity could not be resolved in the improvement of the VAS score and time-sustained self-reported cure outcomes, leading to the placebo effect and selection bias. Therefore, this could affect the significance of the results regarding the clinical practice. Third, studies in this meta-analysis were conducted in different waves of the COVID-19 pandemic, and patients had different circumstances.
We recommend more RCTs on the effect of ICS and INC in COVID-19 patients with larger sample sizes and longer follow-up durations. Also, more RCTs should be done to compare ICS and INC treatment with moderate and severe COVID-19 infection in terms of efficacy and safety outcomes.
Conclusion
We concluded that ICS significantly decreases patient LOS, and INC may have a role in improving the smell score. The ICS also enhances recovery from COVID-19-related symptoms. On the other hand, neither ICS nor INC affects mortality rates or time to self-reported recovery. Our conclusions should be interpreted with caution due to the mixed results and small sample sizes of the included studies.
Contributors
All authors contributed to the conception or design of the work, the acquisition, analysis, or interpretation of the data. All authors were involved in drafting and commenting on the paper and have approved the final version.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
All authors have completed the ICMJE uniform disclosure form and declare no conflict of interest.
Ethical approval
Not required for this article type.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
AI Statement
The authors confirm that no generative AI or AI-assisted technology was used to generate content.




.png)
.png)



.png)
.png)
