Introduction
Children with cystic fibrosis (CF) undertake daily airway clearance (AWC) regimens to maintain lung health. Tracheobronchomalacia (TBM) is present in around 40% of children with CF1 and may impact AWC by impeding expiratory flow and cough effectiveness due to dynamic airway collapse.2 Failure to clear airway secretions effectively can delay illness resolution and increase hospitalizations for respiratory exacerbations.3–6 Understanding the impact of TBM on the effectiveness of AWC in children with CF is, therefore, clinically important.
Positive expiratory pressure (PEP) devices are often recommended for AWC in children with TBM with the rationale that they splint airways open during expiration and reduce airflow obstruction. The amount of PEP resistance on the device is chosen by the experienced physiotherapist based on expiration sound and length or by using a manometer to determine expiratory pressures ranging from 5cmH2O to 20 cmH2O.7,8 While this pressure reflects the PEP pressure measured at the mouth, the physiological effect of PEP on the lungs remains unclear.
There is no evidence on the optimal pressure that is most beneficial in PEP therapy for children with TBM or if PEP prevents airway collapse during expiration. A barrier to studies in this area is the lack of objective measures for the physiological effects of PEP that have no harm (e.g., radiation) or are feasible with children.
Electrical impedance tomography (EIT) is a new technology that may be promising in evaluating the impact of PEP on malacic airways. EIT is a non-invasive imaging method without ionizing radiation that constructs lung images based on the electrical conductivity of biological tissue.9 Electrodes placed around the chest wall use surface voltages to measure differing impedance between body tissue and gas, developing a plethysmography of the volume of each inspiration entering the lung and forming a relative image.10 The collection is painless, data is obtained each second, and analysis can occur in real time.
Most studies involving EIT have explored titration and weaning of ventilation pressures and positioning in sedated, intubated adults within critical care units.11 Studies in non-sedated patients are beginning to emerge with challenges outlined regarding movement artifact, software interpretation related to variable tidal volumes and patient physiological differences.12–14 The use of EIT as an objective outcome measure for PEP airway clearance in non-sedated ambulant children is novel. This is a feasibility proof-of-concept study exploring the potential for EIT to assess physiological changes in the airways and lungs during PEP airway clearance for a child with CF and TBM in a clinical setting. We aimed to establish if EIT is a feasible clinical tool to use in non-sedated, typically developing children with cystic fibrosis to guide appropriate PEP for airway clearance. Efficacy was not measured.
Patients and methods
This study had approval from the Child and Adolescent Health Service Human Research Ethics Committee (RGS0000006376) and was undertaken between April and June 2024. Investigators were physiotherapists with 35 years of combined experience with AWC in children with CF. Draeger Australia® provided EIT training, and investigators undertook 40 hours of familiarisation device training prior to study commencement.
Participants were recruited from a tertiary paediatric hospital CF clinic. Children with a confirmed CF diagnosis were eligible if they: (i) were aged 12 months or older, (ii) had TBM identified on bronchoscopy, and (iii) were using/had used PEP therapy for airway clearance. Children were excluded if they had a respiratory exacerbation requiring intravenous antibiotics in the two weeks prior to the study visit to limit possible acute lung pathology; current haemoptysis, history of pneumothorax or dependency on non-invasive ventilation or oxygen. Eleven children were invited to participate as a sample of convenience who were known to the investigators to match recruitment criteria, lived within commutable distance and were adherent to regular AWC. One child declined secondary to time constraints. Written consent (parent) and verbal assent (adolescent) were obtained. Measures were collected in an outpatient setting during one study visit.
Primary outcome measures in this cohort were: 1) children’s tolerability to the monitoring, 2) ability of the investigators to differentiate between expiratory pressures during AWC using EIT software and 3) ease of EIT device adaptability and administration for the clinical space. Table 1 outlines the criteria against which the feasibility and tolerability of EIT in children were measured and determined.
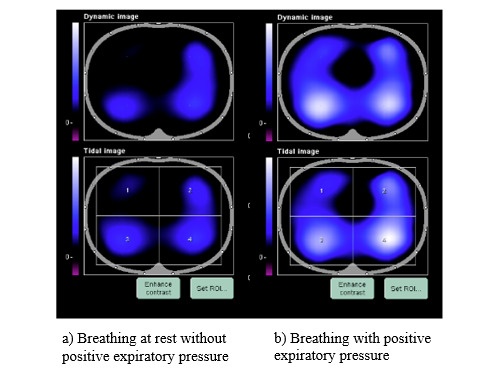
The EIT device chosen for the intervention was the PulmoVista 500 (Draeger ®), an Australian therapeutic goods association-approved device provided gratis on loan by Draeger PTY LTD Australia. The PulmoVista was chosen because electrodes are embedded within a silicone belt, which was anticipated to be easier to apply than individual electrodes, could be cleaned in accordance with hospital and CF-specific infection control protocols, and included small belt sizes to fit infants. A water-based conductivity gel was used to enhance contact between the chest wall and belt. The PulmoVista software provides detailed analysis with graphics on lung regional ventilation flow, distribution and compliance across four regions of interest (ROI): dorsal and ventral, left and right (Figure 1). In analysis mode, recommendations can be seen related to lung compliance and ventilation delay. Software modelling is based on the ventilated patient, where lung volumes and airway pressures are known, and images and software output can be exported for file saving and analysis.
Calibration of the EIT device occurred prior to the participants’ arrival by the two investigating physiotherapists conducting the study (JD and CB). Prior to and at visit commencement, age-appropriate EIT familiarisation was undertaken. On arrival, a general health and AWC interview, along with preparation for belt size and application, were conducted (see supplementary material). Children brought in their own PEP devices. Different resistances were applied by investigators to the PEP device. Pressures were assessed using a manometer to determine and record the resistance level required to achieve pressures of 5, 10, 15 and 20 cmH20 during mid-expiration. All PEP devices used in this study were flow-dependent. In younger children, the estimate closest to these values was chosen due to variability in tidal volume as expected in young children. Participants were monitored by EIT for two minutes tidal/rest breathing without resistance, followed by a total of 16 minutes of AWC, which included eight minutes of breathing against four different resistances (two minutes at each resistance level followed by two minutes rest with no resistance) (see supplementary material). Assistance with resistance changes, timing and technique coaching was given by the investigating physiotherapists. Older children were encouraged to focus on PEP targets by visualizing the manometer. Children performed AWC in their usual position and with normal distraction techniques such as screen time (Table 2).
Demographic data, regular AWC and bronchoscopy descriptions of TBM and structural lung disease were collected. Ability to achieve PEP resistance and complete AWC cycles during intervention was recorded. The quality of the AWC technique was rated by the collaboration of the investigators as either excellent (85% or more), good (50-84%) or poor (less than 50%) based on the estimated percentage of effective PEP breaths over two minutes that met international technique recommendations.8 At completion, investigators recorded their impression of the ideal PEP resistance for AWC based on both clinical assessment and recommendation by clinical interpretation of EIT software analysis.
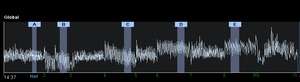
Analyses using EIT software occurred immediately after AWC to assess the feasibility of data collection and are expanded on in Table 1 and supplementary material. Markers (A, B, C, etc.) representing PEP breathing at different resistances were determined both automatically using EIT software and manually by clinicians (Figure 2). Each marker consisted of five breaths per section selected in the last minute of breathing. Software data was collected and analysed in four ways i) dynamic tidal images of region of interest (ROI) and percentage distribution across quadrants ii) positive end expiratory pressure trial recruitability recommendations iii) manual comparison of compliance win and loss in customisation graph analysis; and iv) by review of negative or positive movement of the end expiratory lung impedance (EELI). These four measures were collected to determine the feasibility of collecting these outcomes in non-sedated children for future efficacy trials on preferential ventilation and PEP pressure selection using EIT. Further details regarding specific data collection are described in Table 1. Investigator comments and time taken for analyses were recorded.
For each participant, the investigators scored yes/no for criterion around each of the main outcome measures. To determine if EIT was feasible/ tolerated in non-sedated, typically developing children, criteria were around patient and family tolerability and completion of the intervention. To determine if EIT was able to detect regional ventilation changes in the lung in response to positive expiratory pressure, we looked at criteria related to ROI display and software data output. To determine if EIT was a feasible bedside tool, we examined criteria related to ease of administration and compatibility for the clinic. Criterion are outlined in more detail in Table 1. A positive score of 70% or more across the cohort in a criterion was predetermined to be a measure of success by clinician consensus agreement.
Children were offered a short break, to return at another date/ time more convenient, or to withdraw from the study if behaviour did not enable data collection. These outcomes contributed to the tolerability assessment. Participants were invited to offer feedback on their experiences at the completion of the study visit in an informal and unstructured interview by the investigators related to the tolerability of wearing the chest belt, the length of assessment time, the acceptability of conducting an EIT assessment in the clinical space and the frequency at which it could be undertaken.
Data are expressed using descriptive statistics (means and minimum to maximal ranges). Categorical data are reported as frequencies and proportions. Efficacy was not investigated.
Results
Ten participants recruited had an age range from 15 months to 15 years (mean 6.5 years; 7 children under age 5; 7 males). All had bronchoscopy reported tracheomalacia, two had additional bilateral bronchomalacia, four had left, and one had right bronchomalacia. Seven of the 10 had a bronchoscopy or CT available in the previous 12 months that identified persistent malacia. Eight (80%) children performed airway clearance with a mask. Home airway clearance, PEP resistance, and positioning are outlined in Table 2.
Individual results and group means for all individual criterion against which feasibility was determined are presented in Table 1. Criterion met success for tolerability (mean 98%; range 86-100) and intervention completion (mean 95%; range 90-100). Regions of interest display (mean 96%; range 80-100) and software data analysis (mean 96%; range 90-100) allowed regional lung ventilation changes to be observed with different pressures. Ease of administration and compatibility for the clinic highlighted difficulties with automated software functionality and clinician time (mean 66%; range 10-100%) and clinician time (mean 75%; range 0-100%).
Airway clearance technique
All 10 participants completed AWC with EIT monitoring within 60 minutes. The technique was rated as good to excellent for more than 85% of breaths in 9/10 children. Nine participants were able to generate four different PEP-resistant cycles for assessment. One child refused a fourth resistance cycle. Four children under five years were unable to either generate 20cm H2O or perform the breath cycle for long enough to assess at this pressure. Three had their highest resistance breath cycle terminated early by the investigators due to increased signs of work of breathing. Three of the 10 participants were tested in positions other than sitting (Table 2).
Belt application
Four of the 10 participants had their belts fitted by parents, three younger-aged related to shyness and one adolescent girl for modesty preservation. All reported the application straightforward. Younger children took longer to familiarise themselves with the belt. Two young children indicated that the abdominal electrode sticker was the most irritating factor. Electrodes and/or belt were disconnected during the intervention and were reattached in two children. The belt fit of the 3XS and 2XS sizes was cramped by the large electrode attachments relative to spacing, leading to bunching of electrodes. In these children, placement in the correct anatomical location was difficult due to age-related abdominal protrusion and the proportion of chest wall to abdominal wall space. Two older children required the use of towel padding in the posterior spinal region when sitting supported in a chair to maintain chest wall contact of posterior electrodes. One child had an anterior chest sulcus requiring multiple repositions of the belt to maintain chest wall contact.
Calibration and electrode contact
Calibration took longer than expected in four children due to electrode contact issues; however, all participants achieved high signal quality in 85% of the active breathing investigation time. Multiple EIT device calibrations were required from setup to completion of the intervention (median 2 per participant; range 1-6) after prompting from the device.
Software analyses
In eight of the 10 participants, during initial rest breathing without resistance, there was an ROI identified that proportionately received the least amount of ventilation and an area that received the most. The location of this ROI was variable across participants (Table 1). Trends towards equalization of ventilation display and percentages across ROI were seen when PEP resistance was added by the clinician without child position or belt movement (Figure 3). One child showed initial improvement in a minimally ventilated ROI, but with increasing PEP, this area again reduced. Ventilation ROI % changes were greater in children during prone and/or side lying with PEP than in sitting.
Time taken post-intervention for the software to accept five marker points (i.e. A, B, C, etc, Figure 2), each with a section of 5 breaths, was time-consuming. The software diagnostic recruitability functions did not always accept the section of breaths over the two minutes that the investigators wished to compare to a reference point. At times, the marker point would not set, but on repeated attempts was accepted. The time to achieve this varied greatly from five minutes in one child to more than 45 minutes in five others. Breathing at rest was the most difficult marker to set and was likely because the children were more likely to change their ventilation patterns during this time. The EIT software for automatic placement of markers functioned in only one child, requiring manual placement of markers for analysis in all others. All children had four or more breath points for comparison analysis, but the investigators did not always feel the most ideal breaths were included/accepted with this function. Positive end-expiratory pressure recommended values were collected from automatic software analysis for all patients. Software compliance analysis indicated the lowest compliance was at rest breathing or at the lowest PEP resistance, with greater compliance when resistance was added.
Investigation time
Total time for investigators to complete the set-up, intervention and initial analysis was between 2.5 and 5 h per participant. The maximum time a parent or child participated in the study was 60 minutes.
Discussion
This study demonstrated that the use of EIT is feasible and tolerable in non-sedated, typically developing children when performing AWC. It has the potential to be a useful clinical tool for guiding positive expiratory pressures for AWC; however, further experience with interpretation of software analysis and clinical application is required.
All children had improvements in ventilation volume and compliance with the addition of PEP. Software and ventilation displays showed children had their lowest volume ventilation and greatest compliance loss during resting breathing with no resistance. A heterogeneous ventilation pattern at rest was easily visualized on the device monitor by investigators, older-aged participants, and caregivers of younger-aged participants. Likewise, trends towards homogeneity of ventilation with the addition of PEP could be seen over the four ROI, with changes easier to visualize between larger pressure differences, such as 5 and 15 cmH20, compared to 5 and 10 cmH20. Families reported they found ROI a useful biofeedback tool, and this was the main reason all families felt EIT may be useful in the clinic. Research to investigate if this heterogeneous ventilation pattern is associated with TBM or normal paediatric ventilation distribution, structural lung disease and/or mucus plugging is warranted.
Our results support that regional ventilation changes related to positioning can be examined with EIT, as seen in Figure 4. Although preferential ventilation related to positioning is a cornerstone in physiotherapy AWC practice, little research has been undertaken in this space in children due to ionizing radiation exposure associated with other imaging modalities.15 Belt placement was able to be maintained securely in those patients changing position, but validation studies are required to understand the impact of these changes on software analysis. Establishing ventilation patterns with positioning in both healthy and diseased lungs of non-ventilated children is an exciting future direction for EIT.
Airway clearance with PEP is described in adult studies as effective due to an elevation in functional residual capacity over a cycle of 12-15 breaths.8,16 In our study, some children demonstrated a response in their end expiratory lung impedance (EELI), which is representative of functional residual capacity, within a few breaths of PEP, whilst others required up to a minute to elevate EELI. Likewise, on completion of a PEP cycle, some children had an immediate return of their EELI to baseline, whilst others took considerable time for the effect of PEP to resolve. This representational elevation of functional residual capacity could be seen in some children at 5 cmH2O, whilst others did not achieve it until 20 cmH2O. In four children, the initial application of low levels of PEP showed a reduction of EELI from baseline rest breathing in graph format, as seen in the example in Figure 2. The ability to use the EIT software in the alternative breath-by-breath analysis mode would be helpful; however, in smaller children, we found this trend too variable to interpret for a clinical setting. In the software recruitability mode, the trends of percentage gain or loss in changes of EELI from a selection of breaths at each PEP resistance compared to a reference point may be more useful to examine in future research. If validated, interpretation of EELI could be a promising use of EIT in AWC studies, allowing the clinician to individualize the length of cycles and pressures to gain a desired result in functional residual capacity. EIT could be a non-invasive, well-tolerated tool for the exploration of the physiological response of PEP in children in research.
Most children five years and under struggled to achieve targeted pressures with a flow-dependent PEP device. This is related to the developmental ability to maintain a consistent expiratory flow and volume to achieve a stable targeted pressure. Threshold resistance devices could be considered in future studies to limit the impact of breath flow variability in young children. Likewise, comparison between smaller increments of expiratory pressure in younger children could be beneficial, given that they are less likely to achieve consistently high pressures with their smaller tidal volumes. In this study, all participants were able to tolerate an increase in PEP with AWC but not necessarily the targeted pressure for the study. We also found that gel was required for all children, which has not been reported for non-CF volunteers. This may be due to skin conductivity affected by the presence of salt on the skin in children with CF.
The physical differences in very young children with protruding abdomens and a smaller chest wall to abdomen ratio made belt placement more challenging, with a greater contribution of an abdominal breathing pattern noted in the younger children. Both issues may have affected software analyses and have recently been explored in neonatal populations.12 A recent study suggests it is possible for diaphragm belt placement using ultrasound guidance.17 This could optimize belt placement in children, particularly when considering the impact of heart/chest wall ratios and thymus on imaging interpretation in children. Regardless, in our youngest participants, we were still able to implement the technology, undertake airway clearance for the assessment period and achieve software data output. Further studies are required to assess if, in these very young children, software output correlates to physiological changes in those with very small belt sizes. Although we were concerned that adolescent females may find belt placement problematic, this was not a concern for our single female adolescent participant.
In younger children, EIT software analyses recommendations for recruitability and compliance with PEP needs to be carefully monitored clinically. The greatest compliance gain compared to no resistance breathing was often at the highest trialled pressure, which resulted in excessive work of breathing in three children, and cessation of AWC. These pressures were clinically too high, likely to lead to respiratory fatigue and hinder secretion movement. Whilst an element of malacia may have contributed to participants’ respiratory effort, gains in compliance and ventilation were still seen at lower positive pressures without respiratory effort and would be more ideal. This likely means that although overall software conclusions for recommendations of positive end expiratory pressure could be collected, using this value as a surrogate alone for determining PEP resistance in spontaneously breathing children is not possible.
We believe this study is the only known published data on the use of EIT during PEP therapy in spontaneously breathing children. It supports a recent publication by Moersdorf et al.13 investigated the feasibility of EIT in spontaneously breathing children before and 30 minutes after a range of AWC techniques in 25 inpatients. They did not include PEP therapy, but also concluded it was feasible to monitor changes in regional ventilation distribution in this cohort.
Limitations
Limitations of EIT in this study were a result of cohort age and spontaneous breathing patterns, making clinical interpretation of EIT data necessary over automated software analyses. It is likely that ongoing research and software development would provide improvements over time. If we were to repeat the study in the same children over time, belt position and ventilation volumes would be challenging to replicate. This could limit EIT use for repeated objective value measurement and between-patient comparisons as previously suggested.18 However, it has utility as a biofeedback tool since it is portable, offers no harm and has visual outputs which can easily be interpreted by children and families. We found no limitations with regard to embarrassment of belt placement; however, this cohort has received multiple healthcare interventions requiring exposure of the chest, and the same tolerability may not be reported by non-CF children. Time to complete the software analysis could be considered a limitation. We felt participant study time was acceptable (less than 60 minutes). Although the investigators spent 20 hours training with EIT software analysis, more experience is likely to have improved interpretation time. Time taken for data collection would not be as onerous in clinical practice. Regardless, with ongoing software improvements, there are significant, interesting areas for future research using EIT for optimizing airway clearance routines for children with lung disease and/or illness, with a summary of suggestions offered in Table 3.
Conclusion
In summary, electrical impedance tomography has potential as a feasible tool for guiding airway clearance interventions in non-sedated, spontaneously ventilating children with CF and TBM from the age of 15 months. It shows promise as a useful tool in assisting positioning for ventilation distribution, adjustment of device pressures, determination of length of treatment, and as a visual feedback mechanism for families using a region of interest display and changes in compliance and end-expiratory lung impedance. Further research is required to establish EIT’s accuracy, reliability and repeatability, particularly with regard to motion artifact in spontaneously breathing children.
Funding
We acknowledge Perth Children’s Hospital Parker Physiotherapy Research Fund for assistance with funding support of this project, and Draeger Australia for the loan of EIT equipment during the study.
Competing interests
All authors have completed the ICMJE uniform disclosure form and declare no conflict of interest.
Ethical Approval and Consent to Participate
This study was approved by the Child and Adolescent Health Service Human Research Ethics Committee (RGS0000006376).
AI Statement
The authors confirm that no generative AI or AI-assisted technology was used to generate content.

_rest_breathing_and_(b)_breath.jpg)


_rest_breathing_and_(b)_breath.jpg)