Introduction
Coordinated activation of the diaphragm and extra-diaphragmatic inspiratory muscles is required for efficient inspiration.1 During quiet breathing in healthy individuals, the diaphragm, together with the scalenes and parasternal intercostals, is recruited.1 However, increasing ventilatory demand, such as during exertion or in the presence of respiratory loading due to disease, additional muscles, including the sternocleidomastoids, are recruited to generate greater inspiratory pressures to maintain ventilation.2–7 This increased muscle recruitment is also associated with greater perceptions of dyspnea.2,3,8
Surface electromyography (EMG) provides quantification of both the amplitude (via root mean square: RMS) and the timing (onset and duration of activity relative to inspiratory flow) of inspiratory muscle activation.9–16 EMG timing patterns are shaped by both anatomical and consequent mechanical contributions to chest wall motion, and/or task demands.1,13,15,16 For example, the diaphragm, when recorded by needle EMG, activates earlier than the intercostals and scalenes during quiet breathing13; moreover, the recruitment order of intercostals differs across interspaces.9,15 Further, the scalenes are shown to be activated earlier than the sternocleidomastoid during isovolumetric and dynamic inspirations at different lung volumes.16 Timely activation of these muscles is critical for maintaining ventilatory efficiency.3,9–13
Inspiratory threshold loading (ITL) sustained at a constant load of 50% of maximal inspiratory pressure (MIP) demonstrated no change in the onsets of inspiratory muscles throughout durations to task failure (TF).14 The costal diaphragm/intercostals (measured by surface EMG over the 7th intercostal space) appeared later than scalene, parasternal intercostal and sternocleidomastoid.14 Incremental ITL can more closely mimic real-world conditions such as progressive inspiratory loads during short bouts of exertion or respiratory decline in disease.2 However, the patterns of inspiratory muscle timing and the link between inspiratory muscle timing and outcomes such as inspiratory mouth pressure (Pm) generation, endurance time to TF, and dyspnea intensity remain unclear. A better understanding of these timing relationships during incremental ITL in healthy adults provides a physiological reference for interpreting compensatory muscle behaviour in those with respiratory conditions with altered ventilatory mechanics.
The aim of this study was to evaluate whether the onset timing and duration of EMG activity of different inspiratory muscles vary during incremental ITL in healthy adults. A secondary aim was to evaluate whether the onset timing and duration of EMG activity of inspiratory muscles are associated with greater Pm achieved and dyspnea intensity, and hence longer duration to task failure during an incremental ITL to task failure. We hypothesized that: 1) onset timing of inspiratory muscles EMG activity would become earlier relative to inspiratory flow during increasing inspiratory loads; 2) earlier activation of inspiratory muscle EMG activity would be associated with higher Pm, albeit higher dyspnea intensity at task failure during ITL.
Methods
Participants
It was carried out in accordance with the Helsinki Declaration guidelines and approved by the Ethics Board of the University of Toronto Health Science Research (40294, approved on November 27, 2020). Twelve healthy adults (six males and six females) were recruited through poster advertisements in the university and email announcements distributed to students and working staff in the University of Toronto. Inclusion criteria were: 1) healthy adults aged between 18 and 45 years; 2) body mass index below 30 kg/m2. Exclusion criteria were: 1) current or history of respiratory, cardiovascular, or neurologic diseases that would interfere with testing; 2) competitive elite athlete at the university or national level; 3) pregnancy; 4) either active or ex-smoker; 5) history of surgery or hospitalization because of respiratory, cardiovascular or neurologic symptoms; 6) skin sensitivity to adhesives (i.e., EMG electrodes).2,17–20 An upper age limit of 45 years was applied due to the greater risk of comorbidities at age 5021,22 and elite athletes were excluded due to their higher prevalence of unreported airway hyperreactivity and potential altered physiological and perceptual perception of respirator muscles.23 Participants were screened via telephone with the American College of Sports Medicine screening questionnaire, in addition to confirming whether they met the inclusion criteria prior to scheduling a laboratory visit. This was a secondary cross-sectional analysis of a previously published study conducted between October and November 2022 in a laboratory setting at the University of Toronto.17 Written informed consent was obtained from all participants after explaining the study.
Experimental protocol
On test day, age, height, mass, spirometry and MIP were assessed. Forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were measured using a portable spirometer (COPD-6, Vitalograph, Ennis, Ireland) and FEV1/FVC were calculated in accordance with the standard methodology.24 MIP was evaluated by using a respiratory force meter (MicroRPM, CareFusion, Kent, UK) by instructing participants to perform a maximal inspiration at residual volume.25 MIP was reported in absolute values (cmH2O) and as percent predicted.26 Spirometry and MIP were measured 3 to 8 times as per American Thoracic Society recommendations, and the test was deemed acceptable when the highest three values were within 5% for spirometry24 and 10% for MIP, respectively.25
Next participants had their chair and table heights adjusted so they were seated comfortably with their back straight, facing forward with feet on the floor, and forearms resting forward on the table. While wearing the nose clip, the participant then sealed their lips around the mouthpiece connected to a two-way non-rebreathing valve (1410, Hans Rudolph, Kansas City, USA) with the inspiratory port connected to a pneumotach (3813, Hans Rudolph, Kansas City, MO, USA) (Supplemental Figure 1) in line with a threshold loading device,2,18,19,27 and breathed against loaded device. Participants were familiarized with threshold loading by breathing against an inspiratory trainer (Threshold™, Cardinal Health, Ontario, Canada) before the test session, and considerable efforts were made to adjust the table height and to position the chair and mouthpiece to optimize comfort for the participant. Incremental ITL began with a low warm-up followed by 50 g increments every two minutes until TF.17 TF was defined as either the person withdrawing from the mouthpiece or their inability to lift the threshold valve to sustain adequate inspiratory flow for two consecutive breaths.2,18,25,28 ITL time from warm-up to task failure was defined as endurance time (tlim). Blood pressure, heart rate, dyspnea intensity and dyspnea descriptors were evaluated before and just after ITL.
Outcome measurements
The continuous ventilatory parameters (inspiratory flow, respiratory frequency: fR, inspiratory time: Ti, tidal volume: VT, Pm, and partial pressure of CO2 expressed as end-tidal CO2 (ETCO2) were acquired by using previously described devices and methods (Supplemental Figure 1).2,14,18,19 Participants breathed through a flanged mouthpiece connected to a two-way non-rebreathing valve. The pneumotach measured inspiratory flow, which was utilized to determine breathing frequency, tidal volume, and minute ventilation. Continuous measures of Pm and ETCO2 were recorded from a port close to the mouthpiece via a pressure transducer (MP45, Validyne Corp, Northridge, CA, USA) and carbon dioxide analyzer (17630, VacuMed, Ventura, CA, USA), respectively. Pm was expressed in cmH2O and later normalized to MIP. All ventilatory parameters were sampled at 1000 Hz through a data acquisition system (PowerLab and LabChart 8, ADInstruments).
Surface EMG (Ultium sensor system, Noroxan, Scottsdale, AZ, USA), sampling of 1000Hz, gain of 500, was measured over the scalene, parasternal intercostal, sternocleidomastoid, and costal diaphragm/7th intercostal (Dia/IC) at baseline, during a vital capacity maneuver and throughout incremental ITL. Before attachment of the EMG electrodes, the skin was prepared with brisk rubbing of an alcohol swab and shaving if required. Paired EMG electrodes with a 2 cm inter-electrode distance (Duotrode, Myotronics, Kent, WA, USA) were placed on the right neck/hemithorax over: 1) scalene, in the posterior triangle of the neck at the level of the cricoid process; 2) parasternal intercostal, second intercostal space lateral to the sternum; 3) sternocleidomastoid, midway between the suprasternal notch and the mastoid process; 4) Dia/IC, the 7th or 8th intercostal space between the anterior-axillary line and mid-clavicular line based on the best signal capture (Supplemental Figure 1).2,14,26 The signals of EMG and electrocardiogram (ECG) (BioAmp, Model FE231; ADInstruments) were converted into digital signals. There is a known delay of 300 ms between the point of activity measurement at the Ultium EMG sensor electrode to the moment that the analog outputs are streamed through the PowerLab and LabChart 8 software. Since the EMG onset of inspiratory muscle was analyzed relative to the onset of inspiratory flow—both time-synchronized via LabChart 8—this delay could have introduced systematic timing errors in our analysis. To ensure accurate temporal alignment between EMG and inspiratory flow signals, a time offset of -300 ms was implemented to all EMG signals to correct the delay of information coming through the analog outputs and then stored by data acquisition software (PowerLab and LabChart8, ADInstruments). This correction allowed us to more accurately determine the true onset timing of muscle activation relative to inspiratory flow.
Dyspnea intensity was assessed with the 10-point Borg scale29 and qualitative evaluation of dyspnea was rated by the selection of dyspnea descriptors.30,31 Borg scale was measured at baseline and immediately after task failure, and dyspnea descriptors were assessed only after task failure. Participants were asked to select any of 15 phrases that matched their breathing discomfort at the moment of TF during the ITL31 and to identify the top three descriptors of breathing discomfort.32 There was no restriction on the number of phrases that could be selected.
Algorithm for surface electromyography analysis
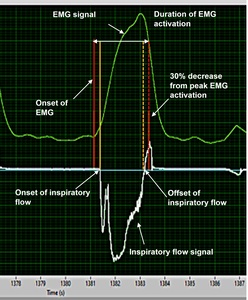
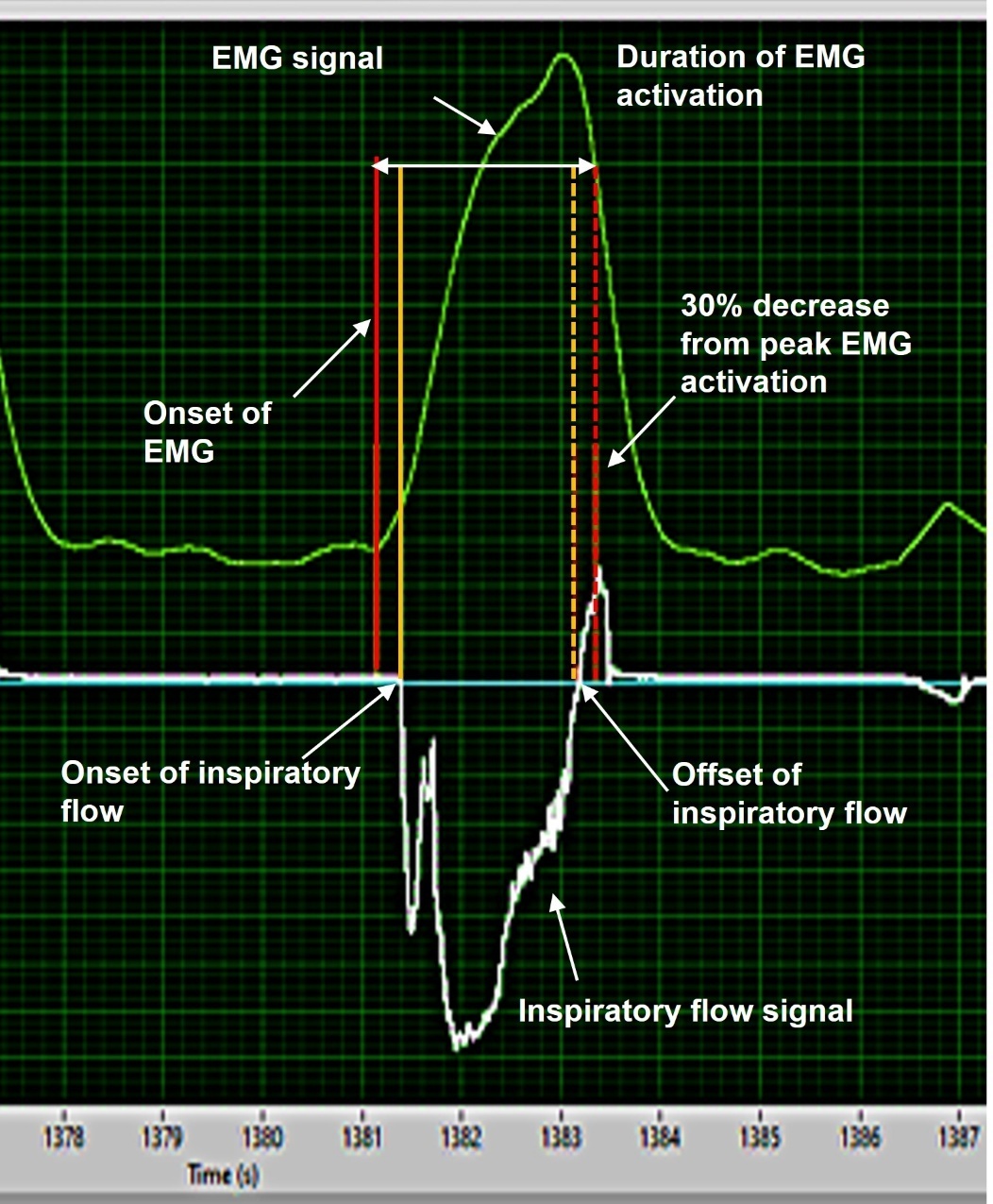
EMG, ECG and inspiratory flow signals were time aligned and imported via txt file from data acquisition software into the algorithm software (LabVIEW, National Instruments, Austin, TX, USA). The previous validated algorithm was applied separately to the four respiratory muscles to remove ECG artifacts and to detect the onset and offset time of the EMG activities.14,28,33 Briefly, a 20 Hz bidirectional high-pass filter and a 2nd-order Butterworth filter were applied, then expressed as RMS values. Then, the timing of inspiratory muscle EMG activity was determined based on the derivative function of each muscle’s EMG RMS. The onset timing of inspiratory muscle EMG activity was defined as the time point at which the RMS value reached 5% of its peak activation (Figure 1). A 5% threshold was applied to avoid identifying fluctuating baseline signals as the EMG activity in the ITL task.14,28 Duration of EMG activation was calculated using the previously validated way as the difference between the onset timing of inspiratory muscle EMG activity and 30% decrease from its peak activity34–36 as exemplified in Figure 1. The algorithm had good to excellent reliability with visual detection and enabled more than 60 times faster analysis than manual detection.14 If timings of inspiratory muscle EMG activity were not identifiable due to artifacts or muscle activities affected by other movements besides inspiration (e.g., neck movement or exhalation), these were considered as missing values. All signals were double-checked visually to confirm those contaminated with artifacts or other movements.
Data analysis
The pressure–time index (PTI) was calculated as a measure of loading and pending respiratory muscle fatigue.37–40 PTI was calculated by using previously described method37–40:
PTI = (Pm/MIP) × (TI/ total duration of the breathing cycle)
For each breathing cycle, the onset timing of inspiratory muscle EMG activity (corrected for the delay of the signal transmission through the analog output) relative to inspiratory flow onset (in milliseconds) were determined for the four respiratory muscles. The onset timing of inspiratory muscle EMG activity of zero indicated that EMG of the inspiratory muscle and inspiratory flow were initiated at the same time, whereas negative or positive values indicated that onset timing of inspiratory muscle EMG activity preceded or occurred after inspiratory flow onset, respectively.14
For analysis, all data were averaged for each load from the warm-up to isoload and at TF. Pseudo-steady state was not obvious amongst all participants during the last 30 seconds of each stage. Warm-up was defined as the first 2 minutes with the initial low ITL. Isoload was defined as the maximum load that all participants completed for the entire 2 minutes. TF load was defined as the maximum load that each participant was able to complete for 2 minutes.
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS Inc., version 25.0, Chicago, IL, USA). A two-tailed significance level of p < 0.05 was used for all tests. Prior to analysis, the Shapiro-Wilk test was used to examine the normality of the data.
Data are presented as mean ± standard error for EMG and ventilatory parameters since the group data is the mean of means. In other words, the mean data is derived from several breaths for each participant, and then the group mean is calculated from the mean data from each participant. For dyspnea descriptors, frequencies and percentages were reported due to their categorical nature.
Repeated-measures comparisons of inspiratory muscle EMG timing (onset and duration), EMG RMS amplitudes, and ventilatory parameters across the incremental ITL stages (warm-up to isoload, and TF loading) were performed using two-way repeated-measures analysis of variances (ANOVA). Post-hoc tests were performed using Bonferroni’s correction. This test was selected due to its suitability for evaluating within-subject changes across multiple time points and muscle groups when assumptions of normality are met.
In cases where assumptions of normality were violated, non-parametric alternatives were used. Specifically, the Wilcoxon signed-rank test was used to compare Borg dyspnea ratings between baseline and task failure due to the ordinal nature and non-normal distribution of Borg dyspnea scores.
Pearson correlation coefficients were used to assess associations between 1) EMG onset timing and inspiratory effort (measured as Pm and Pm/MIP) and EMG RMS amplitude, 2) EMG parameters (onset, duration, and RMS amplitude) and tlim. Spearman’s correlation was applied to assess associations between EMG parameters and Borg dyspnea intensity at TF load of each muscle since Borg dyspnea did not follow a normal distribution.
Using G*Power 3.1, a sample size of 11 was estimated based on a power (1-β) = 0.80, α=0.05 and an effect size of 0.84 calculated from previous data.2 We aimed to recruit a sample of 12 in order to have equal numbers of males and females.
Results
Descriptive characteristics of participants and incremental ITL
Characteristics of 12 participants are shown in Table 1. They had a mean age of 29.5 ± 7.1 years, with equal numbers of males and females, and were within normal ranges for body mass index, spirometry and MIP, suggesting preserved ventilatory capacity. Participants may have been recreationally active but not involved in competitive athletics. Females were younger and had lower spirometry, both absolute and percent predicted, than males, whereas there were no sex differences in other variables (Table 1). Participants started ITL at warm-up of 7.7 ± 3.5 % of MIP (Figure 2B). The mean tlim to TF was 1012 ± 428 sec (16.9 ± 7.1 min) and the mean TF load was 350 ± 164 g (46.8 ± 16.3% of MIP; Figure 2). No participants stated that they withdrew from the mouthpiece due to discomfort, fatigue of facial muscles, excessive saliva or for other reasons besides failure to overcome the inspiratory load. The isoload (highest load obtained by all participants) was 150 g, which equated to 25.4 ± 11.8 % of MIP (Figure 2B). The correlation between MIP and tlim during inspiratory threshold loading was not statistically significant (r = 0.57, p = 0.054).
Compared to the warm-up, Pm and Pm/MIP were higher at the isoload (p ≤ 0.033) and highest at the TF load compared to earlier loads (p < 0.001; Figure 2A, B). There were no significant changes in fR, Ti, VT and ETCO2 throughout the incremental ITL (Figure 2C, D, E, H) (p ≥ 0.223). PTI increased only at the TF load compared to other loads (p ≤ 0.006; Figure 2G).
The EMG RMS of parasternal intercostal, scalene and sternocleidomastoid increased throughout the incremental ITL (p ≤ 0.016, Figure 2H). RMS of Dia/IC did not increase throughout the incremental ITL (p ≥ 0.241, Figure 2H).
EMG Timings of inspiratory muscles during incremental ITL
A total 1,511 breaths from twelve participants were analyzed (mean 126 ± 61 breaths per participant). The onset timing of inspiratory muscle EMG activity of each muscle was assessed, totaling 6,044 potential EMG activities (1,511 breaths × 4 muscles). On average, 6% of the 6,044 potential EMG activities could not be detected due to movement artifact or low amplitude.
At TF (compared to the warm-up), the onset timing of inspiratory muscle EMG activities occurred earlier relative to the flow for the sternocleidomastoid (p < 0.001), scalene (p = 0.002) and parasternal intercostal (p = 0.002) (Figure 3). In contrast, the onset timing of Dia/IC EMG relative to the flow did not change throughout the incremental ITL (p > 0.05, Figure 3). Activity duration of the EMG signals of all muscles did not change significantly with increasing ITL loads (p > 0.147; Figure 3). The onset timing (p ≥ 0.083) and duration of inspiratory muscle EMG activity (p ≥ 0.189) did not differ among four muscles.
Correlation with EMG timings
Earlier onset timing of the parasternal intercostal, sternocleidomastoid and scalene EMG activities relative to flow were associated with higher Pm/MIP (p ≤ 0.034) (Figure 4) and higher EMG RMS (p ≤ 0.015) (Supplemental Figure 2). Earlier onset timings (relative to flow) of the scalene EMG activity during initial stages of the incremental ITL (warm-up and 50 g) were correlated with achieving a high Pm at TF and a longer tlim (p ≤ 0.026, Table 2). Duration of EMG activities were not associated with tlim (p ≥ 0.308).
Dyspnea sensation
Dyspnea intensity (10-point Borg scale) was greater at TF than at the baseline (5 ± 3 vs. 0.1 ± 0.3, respectively, p < 0.001). Onset timing of the parasternal intercostal and sternocleidomastoid EMG activity relative to flow at TF load was correlated with Borg dyspnea intensity after ITL task failure (r = -0.67, -0.65, p = 0.023 both) (Table 2). Duration of EMG activations and RMS amplitudes of all four inspiratory muscles at TF load were not correlated with Borg dyspnea intensity (p ≥ 0.089) (Table 2). The top two descriptors were within the work/effort cluster (“Breathing in requires more effort”: 66.6% and “My breathing requires more work”: 50.0%). Unsatisfied inspiration (“I cannot get a deep breath in”), Heavy ("My breathing is heavy ") and Inspiratory difficulty (“My breath does not go in all the way”) were the next descriptors (33.3%, respectively).
Discussion
This is the first study to examine timing of surface EMG activities from four inspiratory muscles during incremental ITL to task failure to further define the contribution of the extra-diaphragmatic muscles. Consistent with our hypothesis, increasing ITL load to task failure induced earlier onset timings of parasternal intercostal, sternocleidomastoid and scalene relative to inspiratory flow whereas this onset timing of the Dia/IC relative to flow did not change throughout the ITL. Early activations of parasternal intercostal, scalene and sternocleidomastoid were associated with larger magnitude of the inspiratory effort (Pm) and EMG RMS. Early activations of parasternal intercostal and sternocleidomastoid relative to flow at task failure of ITL were associated with greater dyspnea intensity. Earlier onset timings of scalene EMG activity relative to flow during the earlier stages of incremental ITL were associated with a longer tlim and higher Pm achieved at task failure.
Altered onset timings of extra-diaphragmatic muscles are in contrast to those demonstrated by Rodrigues et al,14 during constant ITL (50% MIP) to task failure when the onset timings of all inspiratory muscle EMG activities did not change throughout the task.14 We began ITL with a low warm-up load (7.7 ± 3.5 % of MIP) followed by the load increasing up to 46.8 ± 16.3% of MIP at task failure, comparable to the constant ITL load applied in the study by Rodrigues et al.14 The physiological implications of altered onset timings of extra-diaphragmatic muscles suggest that earlier recruitment of these muscles at low loads act as a reserve to enable force production against progressively increasing threshold pressures. Early activation in addition to greater recruitment of extra diaphragmatic inspiratory muscles during ITL may serve to optimize chest wall mechanics, reduce the load on the diaphragm, and maintain ventilatory efficiency.16,41 Evaluative and treatment strategies of respiratory muscle function are often centered on inspiratory strength with a primary function on the diaphragm. An appreciation of the coordinated timing of inspiratory muscles in healthy people will provide a foundation for examining the value of treatment approaches in those with respiratory compromise. Thus, our results build on previous studies3,16,41,42 by showing that timing—not just amplitude—of several inspiratory muscles in addition to the diaphragm contribute to endurance during respiratory loading.
The timings of extra-diaphragmatic inspiratory muscles appear to change with increasing load, that is, higher loads lead to earlier recruitment (relative to flow). This does not seem to occur during constant load ITL.14 Since constant load ITL does not necessarily induce muscle fatigue,20,43 this finding does not rule out the possibility that the timing of extra-diaphragmatic inspiratory muscle activations could change in the presence of fatigue - this remains to be studied. What it does suggest is that earlier activation of these muscles may signal that the respiratory system is experiencing increased loads, potentially contributing to a heightened sensation of dyspnea and prompting participants to stop the test. This interpretation aligns with earlier studies showing that premature sternocleidomastoid activation was associated with respiratory failure in mechanically ventilated patients.5
Earlier scalene onset during the initial stages of incremental ITL was associated with achieving longer tlim with greater Pm at task failure (Table 2). Scalenes are recruited for inspiration not only during quiet breathing,16 but also during loaded breathing.3,6,16,43 Scalenes act as a flow reservoir until sternocleidomastoid muscle recruitment increases substantially during increasing inspiratory loads to augment ventilation provided by the primary muscle of inspiration, the diaphragm.3,16 Increased sternocleidomastoid activation reduces the need for scalene activation when ventilatory demands increases.2 Muscle activities of both sternocleidomastoid and scalene increased at TF load (Figure 2H) but onset timings between the sternocleidomastoid and scalene EMG activities were not different. Thus, regardless of recruitment timing of sternocleidomastoid, our results suggest that the complementary contribution of earlier onsets of scalene at low, early loads may influence endurance against higher loads together with the primary contribution of the diaphragm.
The onset timings of extra-diaphragmatic inspiratory muscles appear to change with increasing load, that is, higher loads lead to earlier recruitment (relative to flow). This strategy does not appear to reflect muscle fatigue, as PTI values remained below (Figure 2G) the fatigue threshold 0.15.39 The mean values of PTI in our study did not exceed 0.15 even at TF (Figure 2G). The mean tlim was more than double in the studies that imposed a constant load (50% ± 5% of participant’s MIP) compared to ours (maximum 46.8 ± 16.3% of MIP); mean of 38.1 min ~ 2283 sec20 and 40.1 min ~ 2406 sec43 vs. 1012 sec in our study. However, the duration of our study and type of loading was similar to the mean tlim (mean 17 min ~1020 sec) of another report that found no evidence of respiratory muscle fatigue after incremental ITL to TF.2 This is further supported by the absence of significant change in breathing frequency or tidal volume (Figure 2C, D), suggesting that earlier muscle activations occur independently of gross ventilatory adjustments with increasing inspiratory load. This finding does not rule out the possibility that the timing of extra-diaphragmatic inspiratory muscle activations could change in the presence of fatigue - this remains to be studied. What it does suggest is that earlier activation of these muscles may signal that the respiratory system is experiencing increased loads, potentially contributing to a heightened sensation of dyspnea and prompting participants to stop the test. This interpretation aligns with earlier studies showing that premature sternocleidomastoid activation was associated with respiratory failure in mechanically ventilated patients.5
Dyspnea could be another potential reason for task failure during incremental ITL. Earlier onset timing of parasternal intercostal and sternocleidomastoid EMG relative to flow at TF load were associated with higher Borg dyspnea intensity (Table 2). The top three dyspnea discomforts selected in this study were similar to those chosen by patients with chronic obstructive pulmonary disease (COPD) or interstitial lung disease (ILD).7,27,44 The top two descriptors within the work/effort cluster are consistent with previous findings from threshold loading.45 Unsatisfied inspiration (“I cannot get a deep breath in”), considered to be aligned with air hunger, the uncomfortable or unpleasant urge to breathe,45 was the next most common descriptor. This increase in dyspnea intensity may be attributed to neuromechanical dissociation induced by a rise in respiratory drive with no concurrent increase in Vt.31,45 In summary, incremental ITL can induce earlier onset timings of EMG relative to flow to facilitate stronger inspiratory effort (Pm) (Figure 4) together with recruitment of other respiratory muscles including the diaphragm, to address increased inspiratory loads (Supplemental Figure 2), but also induces higher dyspnea intensity and in some emotional discomfort simultaneously, contributing at least in part to TF.
Clinically, the altered timing of inspiratory muscles observed in this study has several important implications. First, these findings provide a physiological reference for comparison of potentially dysfunctional coordination in respiratory conditions such as COPD or ILD. In particular, the recruitment patterns of scalene and sternocleidomastoid muscles have been reported to be altered in people with COPD to complement recruitment of other respiratory muscles, including the diaphragm.3,6 Further appreciation of the timing in addition to the magnitude of inspiratory muscle activation may enable differentiation between compensatory strategies versus impending respiratory failure. Moreover, timing changes of inspiratory muscles may help to identify individuals’ readiness to wean from mechanical ventilation during a spontaneous breathing trial. It was noted that earlier and greater sternocleidomastoid recruitment of weaning from mechanical ventilation may be a sign of pending failure during a spontaneous breathing trial.5 Our study provides that the timing of scalene activity, in addition to sternocleidomastoid, may influence the ability to endure higher levels of inspiratory loads. In addition, qualitative descriptors of dyspnea during ITL at TF were similar to those expressed by COPD and ILD patients,7,30,44 which provides some inference of the clinical relevance of the incremental ITL stimuli. Therefore, appreciation of inspiratory muscle timing may provide further insight into pending ventilatory failure and interventions that could reverse or prevent respiratory muscle dysfunction to meet increased demands.
The onset timing and RMS of surface EMG Dia/IC did not change throughout the ITL (Figure 2H and Figure 3). The diaphragm is the primary muscle of inspiration and works during quiet breathing.1 Even loaded breathing, diaphragm may work by stabilizing the lower ribcage in addition to generate inspiratory flow.1,6,41,43 Some explanation for the differences in our findings is the surface electrodes were placed over the costal diaphragm/7th or 8th intercostal, not needle EMG. Surface EMG of Dia/IC may include the neural activity of the costal diaphragm and intercostals in addition to influences of fat tissue or skin movement. Although needle EMG may reduce cross talk it is limited to a relatively small number of motor units, poses more risk which limits its application to many participants and patients. Surface EMG, on the other hand, has the potential for more widespread application. Other previous studies have reported that there was little change in the diaphragm, but activity of extra-diaphragmatic inspiratory muscles increased during loaded breathing.1,3,6,16,41–43 Our results appear to be similar to these previous reports. It should be noted that the focus of this report is on the extra-diaphragmatic muscles, which does not negate the vast literature delineating the contribution of the primary muscle of ventilation, the diaphragm.
A potential factor that may affect the interpretation of results is breathing patterns. It has been noted that inspiratory muscle fatigue, which may influence task failure is affected by breathing frequency and duty cycle.2 Even though we did not control breathing frequency or breathing pattern during ITL, breathing frequency did not change throughout ITL (Figure 2C). Although we have guided the breathing frequency in previous reports,14,19,24,43 our goal for this study was to further understand and appreciate the variable response that participants exhibit during loaded breathing. The rationale for this approach is because breathing pattern is not controlled for patients in respiratory distress associated with acute or chronic respiratory muscle loading. The variation of breathing pattern in patients may in fact be an important predictor that maintains ventilation or contributes to ventilatory failure or task failure.
A limitation of this study was the small sample of males and females, which limited our ability to determine if sex differences influenced EMG timings and tlim in our sample (six males and six females). Compared to males, females have been reported to have narrower airways, smaller lung and rib cage dimensions, and shorter diaphragm muscle length.46–48 Further, the contribution of the thoracic and neck muscles (e.g. sternocleidomastoid and scalene muscles) to the diaphragm muscle was greater in females than males.49,50 Moreover, the greater recruitment of sternocleidomastoid and scalene muscles during loaded breathing was observed in females.1 Of interest, greater sternocleidomastoid recruitment was observed in a sample of males who fail to wean from mechanical ventilators.5 Sex differences warrant further investigation, as well as examination of other factors in healthy people and in those with disease that might affect respiratory muscles’ contributions to chest wall mechanics.
Conclusion
This study provides novel findings that earlier activation of extra-diaphragmatic inspiratory muscles—specifically the scalene, parasternal intercostal, and sternocleidomastoid—can be a compensatory strategy to achieve higher loads during incremental ITL in healthy adults. Earlier onsets of extra diaphragmatic inspiratory muscle were associated with achieving larger inspiratory efforts and increased EMG amplitudes suggesting a functional role in maintaining ventilation under increasing load. Onset of scalene in the early stages of the ITL was associated with higher Pm and longer endurance time at task failure whereas earlier onset timing relative to flow of parasternal intercostal and sternocleidomastoid at task failure was associated with higher dyspnea intensity. These results further our understanding of the coordination of respiratory muscles under inspiratory load and suggest that timing—not just amplitude of activation effectively contributes to ventilatory performance and dyspnea perception.
Clinically, these findings provide physiological reference for interpreting altered timings of respiratory muscles in conditions such as COPD, ILD, or during weaning from mechanical ventilation. Earlier recruitment of extra-diaphragmatic muscles may serve as a non-invasive marker of inspiratory load compensation or risk to ventilatory failure. Appreciation of inspiratory muscle timing may provide further insight into understanding the contributors to ventilatory task failure and dyspnea in efforts to forestall or prevent respiratory muscle failure in those with acute and chronic respiratory conditions. Future research should explore how these timing adaptations differ in populations with respiratory disease, assess potential sex differences, and determine whether interventions (e.g., inspiratory muscle training) can modulate timing to improve clinical outcomes.
Funding
This work was supported by a National Institutes of Health (NIH) grant (R21EB031250). AR is supported by a Canadian Institutes of Health Research (CIHR) Fellowship (#187900).
Competing interests
All authors have completed the ICMJE uniform disclosure form and declare no conflict of interest.
Ethical Approval and Consent to Participate
Approval was granted by the Ethics Committee of University of Toronto Health Science Research (#40294). Written informed consent was obtained from all participants after explaining the study.
AI Statement
During the revision of this work, the authors used ChatGPT to proofread English (word choice, grammar, fluency). After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Data availability
Data available on request due to privacy/ethical restrictions.

_root_mean_square_(rms)_amplitude_during_.jpeg)
_relative_to_inspiratory_flow_(panel_a)_and_duration.jpg)
_and_mouth_p.jpg)
_root_mean_square_(rms)_amplitude_during_.jpeg)
_relative_to_inspiratory_flow_(panel_a)_and_duration.jpg)
_and_mouth_p.jpg)