Introduction
Scientific-technological advances in neonatal intensive care have increased the survival of premature newborns1; however, other comorbidities related to prematurity have emerged, such as bronchopulmonary dysplasia (BPD)1,2 related mainly to the interruption of distal lung growth.3 Its pathogenesis remains unclear, and the definition is still imprecise and varies among institutions and evidence-based guidelines.4 One of the definitions of BPD considers mild BPD infants who have received oxygen or respiratory support for >28 days but are breathing room air at 36 weeks postmenstrual age (PMA). Babies with moderate BPD are those who require supplemental oxygen with a fraction of inspired oxygen (FiO2) concentration <30%, at 36 weeks PMA Finally, severe BPD is classified as use of >30% oxygen or positive pressure at 36 weeks.5 It is characterized by persistent abnormalities in gas exchange, airway function, respiratory system mechanics, and lung volumes.6 These changes cause a prolonged period of hospitalization for these preterm infants in neonatal units.1
During the period of hospitalization in the Neonatal Intensive Care Unit (NICU), premature infants with BPD are exposed to a series of external factors, such as invasive and non-invasive procedures necessary for their monitoring and treatment, but which can promote excessive sensory stimuli.1 These stimuli can trigger a generalized response to stress, including cardiorespiratory, hormonal and behavioural changes. Such physiological responses are followed by endocrine-metabolic reactions with consequent homeostatic and physiological imbalance leading to reduced oxygen saturation and increased heart and respiratory rates.1 Thus, it is necessary to use multidisciplinary therapeutic measures that aim to promote comfort for these premature infants.7
Hydrotherapy is a non-pharmacological intervention used in the NICU for preterm infants across various clinical conditions. By combining the thermal and physical properties of water, it promotes beneficial physiological responses. This technique has been shown to safely and effectively reduce pain and stress, while also improving key parameters, including heart rate (HR), respiratory rate (RR), and peripheral oxygen saturation (SpO₂).7 However, even though the benefits of hydrotherapy in preterm infants have been described, there is no information regarding the effects of this technique solely in preterm infants with BPD. Therefore, this study aimed to evaluate the effects of bucket hydrotherapy on physiological and behavioural parameters, as well as the need for oxygen (O2) use, in preterm infants with BPD during hospitalization in the neonatal unit. Our main hypothesis is that bucket hydrotherapy improves SpO₂ levels and consequently reduces oxygen requirements, while also enhancing respiratory pattern, promoting relaxation, and improving behavioural parameters in children with BPD.
Methods
This is a randomized clinical trial (RCT) registered prospectively in ClinicalTrials.gov (#NCT03538977), carried out at the NICU of the University Hospital of Londrina between June 2018 and December 2019, composed of a control (conventional physiotherapy – PG) and an intervention group (conventional physiotherapy plus bucket hydrotherapy – BHG). The study was approved on November 12, 2017, by the university’s Ethics Committee (#2.377.175).
A free and informed consent form with information about research´s ethical and legal aspects was signed by those responsible for the infants who met the inclusion criteria and agreed to participate in the study. The inclusion criteria were infants born with a PMA between 23 -36 weeks; diagnosed with BPD according to the criteria of Jobe and Bancalari5 classification used in the data collection institution during the period in which it occurred; necessity of O2 therapy by low-flow nasal cannula, invasive mechanical ventilation or non-invasive ventilation (NIV); absence of central venous access, skin lesions, operative wound, drains, heart disease and adrenal insufficiency. The exclusion criteria were infants unable to perform hydrotherapy for three consecutive days due to severe respiratory effort assessed by the Silverman-Andersen Bulletin (SAB) and/or hemodynamic instability, defined as the presence of hypotension, tachycardia or SpO₂ levels ≤90%.
Sample size calculation was based on the study by Vignochi et al7 using the difference and standard deviation of SpO2 before and after hydrotherapy in preterm babies. Assuming an alpha of 0.05, power of 90% and a loss of 30%, the number of randomized babies would be 20 (GPower 3.1.3 software – Franz Faul, Universität Kiel, Germany). Randomization was performed at the beginning of the study, in four blocks of six participants. Each block was balanced with three participants allocated to each group (PG and HG) using www.random.org. Participants’ allocation was granted in sequentially numbered and sealed opaque envelopes. The envelope opened sequence defined the allocation group of each preterm infant. Methodological procedures of randomization and allocation were carried out by a researcher not involved in the study.
Subjects in PG received conventional physiotherapy three times a day, including stretching of respiratory muscles, support in the thorax and abdomen, mobilizations, airway clearance techniques and positioning. Each session lasted around 10 to 15 minutes and was performed by the physiotherapy team from the unit. BHG received two sessions of conventional physiotherapy and one session of hydrotherapy daily. Bucket hydrotherapy was performed in a sterilizable stainless-steel bucket with dimensions of 30 cm in diameter and 32 cm in depth, filled with water. The water temperature was adjusted between 37 °C and 38 °C, measured with a clinical mercury thermometer (5240, Buba Toys®, São Paulo, Brazil). The amount of water used was sufficient to keep the infant immersed up to shoulder height. After the initial assessment, the infant was slowly immersed in warm water and suspended by occipital support, performed by the physical therapist, which allowed the infant to move freely in the water for ten minutes (Supplementary Figure 1). The therapy would be interrupted in case of intense agitation, cyanosis, worsening respiratory distress or defecation.
The therapists responsible for applying bucket hydrotherapy (Darllyana de Sousa Soares and Victoria Cristina Escobar) have experience in neonatal care, and they underwent specific training for 2 months before the study to standardize the use of bucket hydrotherapy. The technique was applied according to the protocol outlined in prior studies.7–9
The intervention took place over 12 consecutive days. Previously published studies7,8 evaluated bucket hydrotherapy over a single day. Since our goal was to assess the effects of bucket hydrotherapy in the short, medium, and long term, we opted for a 12-day intervention, maintaining consistent intervals between evaluation days. The first (D1), sixth (D6) and twelfth (D12) day of intervention were selected for analysis. Assessments were always performed in the morning and at five different moments: immediately before therapy, immediately after, 15’, 30’ and 60’ after the intervention - whether PG or BHG.
Heart rate, SpO2, RR, pain, respiratory effort, sleep, and wakefulness status were measured. A multiparameter monitor (Efficia CM120, Philips®, São Paulo, Brazil) was used to assess HR and SpO2. FiO2 was delivered and measured by a mechanical ventilator mixer while using nasal oxygen cannula or NIV according to individual needs, using the least amount of O2 needed to maintain SpO2 levels between 91-95%.10 The physical therapist visually measured the RR for one minute. A 30-second video was recorded, and subjective variables, including pain, respiratory effort, sleep, and wakefulness, were evaluated by a blind evaluator. The blind evaluator was selected based on her experience in neonatology, having worked in NICU for a minimum of two years and demonstrating familiarity with the assessment scales employed. Additionally, a 15-day training program was conducted to ensure proficiency in the use of these scales.
The assessments were made using specific scales: the Neonatal Infant Pain Scale (NIPS), the SAB and the Sleep and Wake Assessment Scale, adapted by Brazelton.7–9
The NIPS assesses pain based on the following parameters: facial expression, crying, breathing pattern, leg position, arm position, and state of arousal. Pain is considered present when the score is greater than three.11 SAB quantifies respiratory distress evaluating the items: thoracoabdominal synchronism, intercostal retraction, xiphoid retraction, nasal flaring and expiratory groaning. It ranges from zero (no respiratory discomfort) to ten points (maximum discomfort).12 The Sleep and Wake State Assessment Scale, adapted by Brazelton, analyzes the behaviour of preterm infants in relation to the stages of sleep and wakefulness.13 Other clinical data related to the mother and gestational period of the included infants were retrieved from hospital charts.
Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, California, USA) and SPSS 20.0 (Statistical Package for the Social Sciences Inc., Chicago, Illinois, USA) software. Data normality was verified using the Shapiro-Wilk test and was described as mean ± standard deviation or median [interquartile range 25-75%]. Categorical data were analyzed using Chi-square or Fisher´s exact tests. Intergroup comparisons were checked using the unpaired Student’s t-test or Mann-Whitney U test. For intragroup analyses, repeated measures ANOVA or Friedman’s test was used, depending on the data distribution, in addition to Dunn’s multiple comparison test. A 5% level was set as significant. Effect size (ES) (Cohen’s d) calculation was performed for the mean differences between groups. ES of 0.2, 0.5 and 0.8 were considered small, medium and large, respectively.14,15
Results
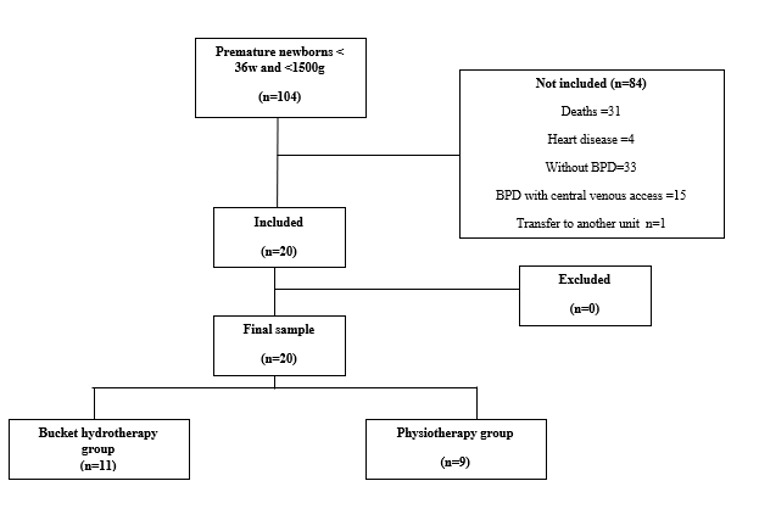
A total of 104 preterm infants (GA <36 weeks and birth weight ≤1500g) were born in the NICU during the study period. Eighty-four were not included due to death (n=31), congenital malformations or heart disease (n=4), absence of a diagnosis of BPD (n=33), use of central venous access (n=15), and transfer to another unit (n=1). Twenty preterm infants with BPD were included, and none of them were lost to follow-up (Figure 1). Table 1 presents the clinical characteristics of the studied population.
The duration of bucket hydrotherapy for all infants in the BHG group was the same, lasting ten minutes. None of them presented intense agitation, cyanosis, worsening of the breathing pattern or defecation during the procedure.
Supplementary Table 1 shows the types of ventilatory support provided throughout the study for both groups. Ventilatory assistance, whether via nasal cannula, CPAP, or invasive mechanical ventilation, was maintained according to each patient’s individual needs. No significant differences were observed between the groups in terms of ventilatory support.
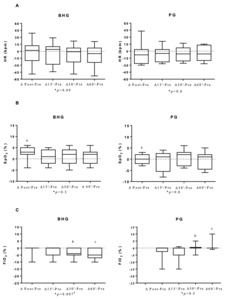
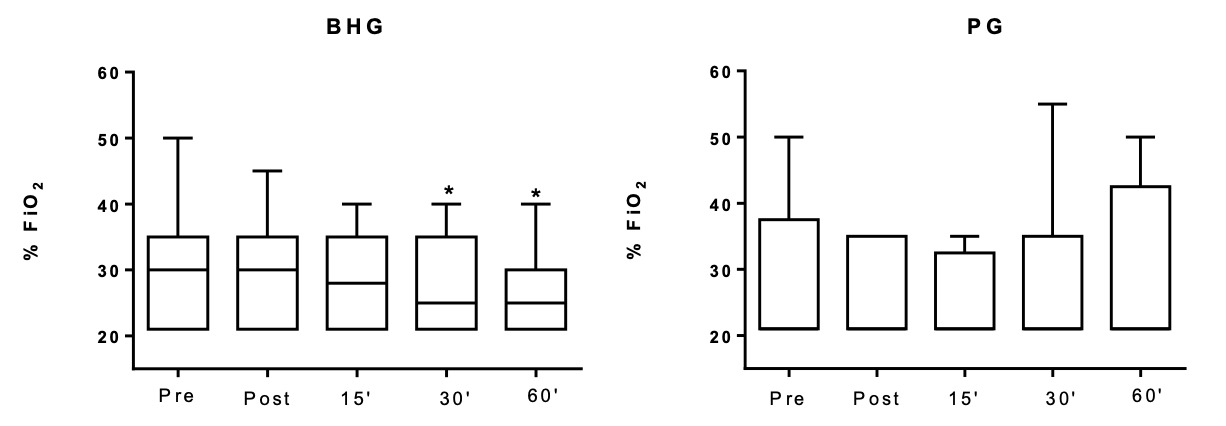
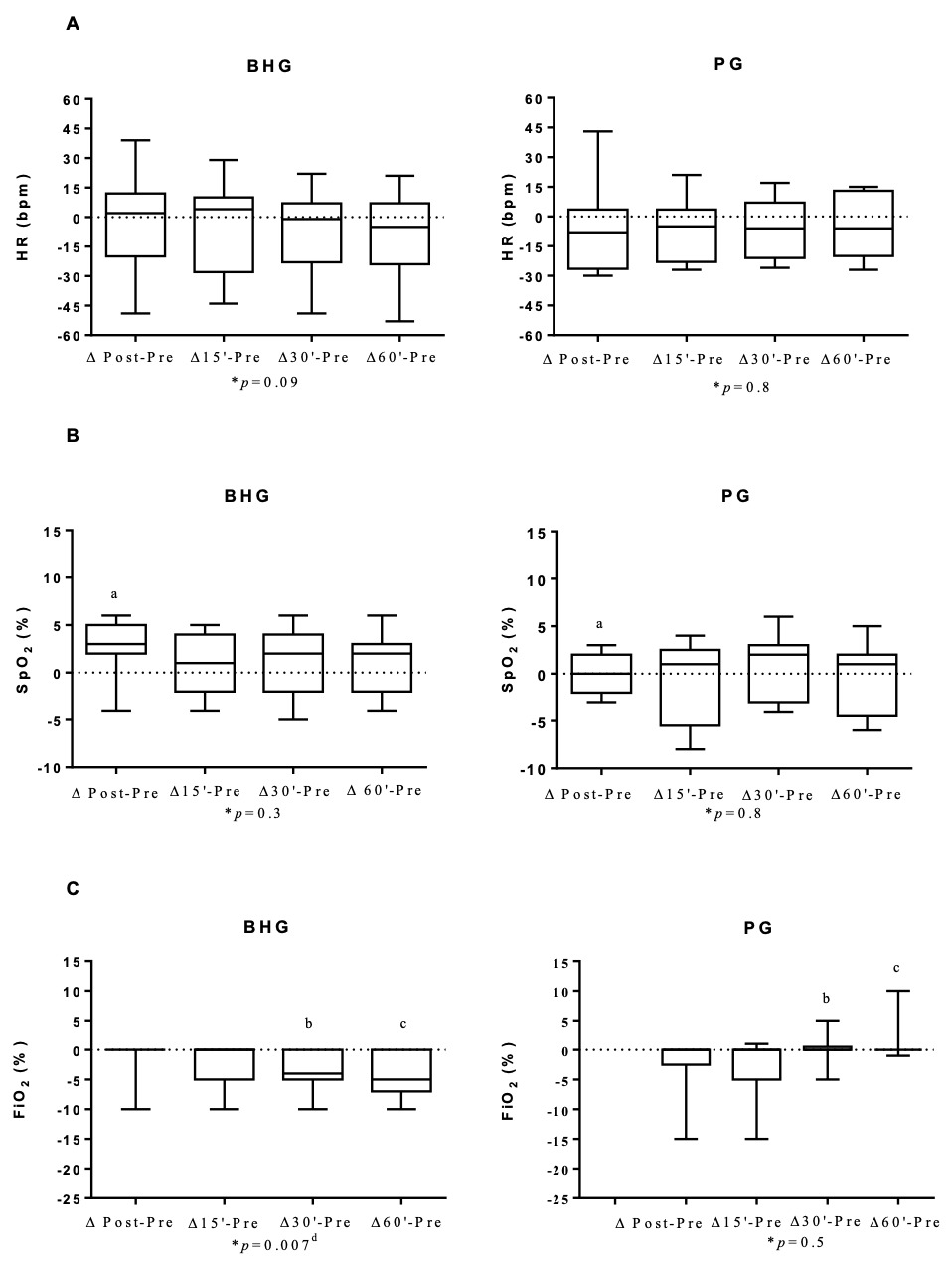
In the BHG, the intragroup comparisons on D1 showed improvement in the FiO2, which was lower at 30’ (25[21-35]%) and 60’ (25[21-30]%) after bucket hydrotherapy, in comparison to before the intervention (30[21-35]%) (p = 0.03 and p = 0.02, respectively) (Figure 2). There were no significant differences in any other studied variables on D1.
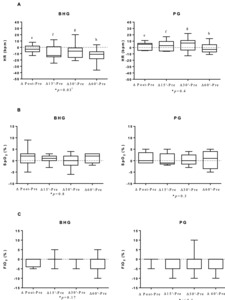
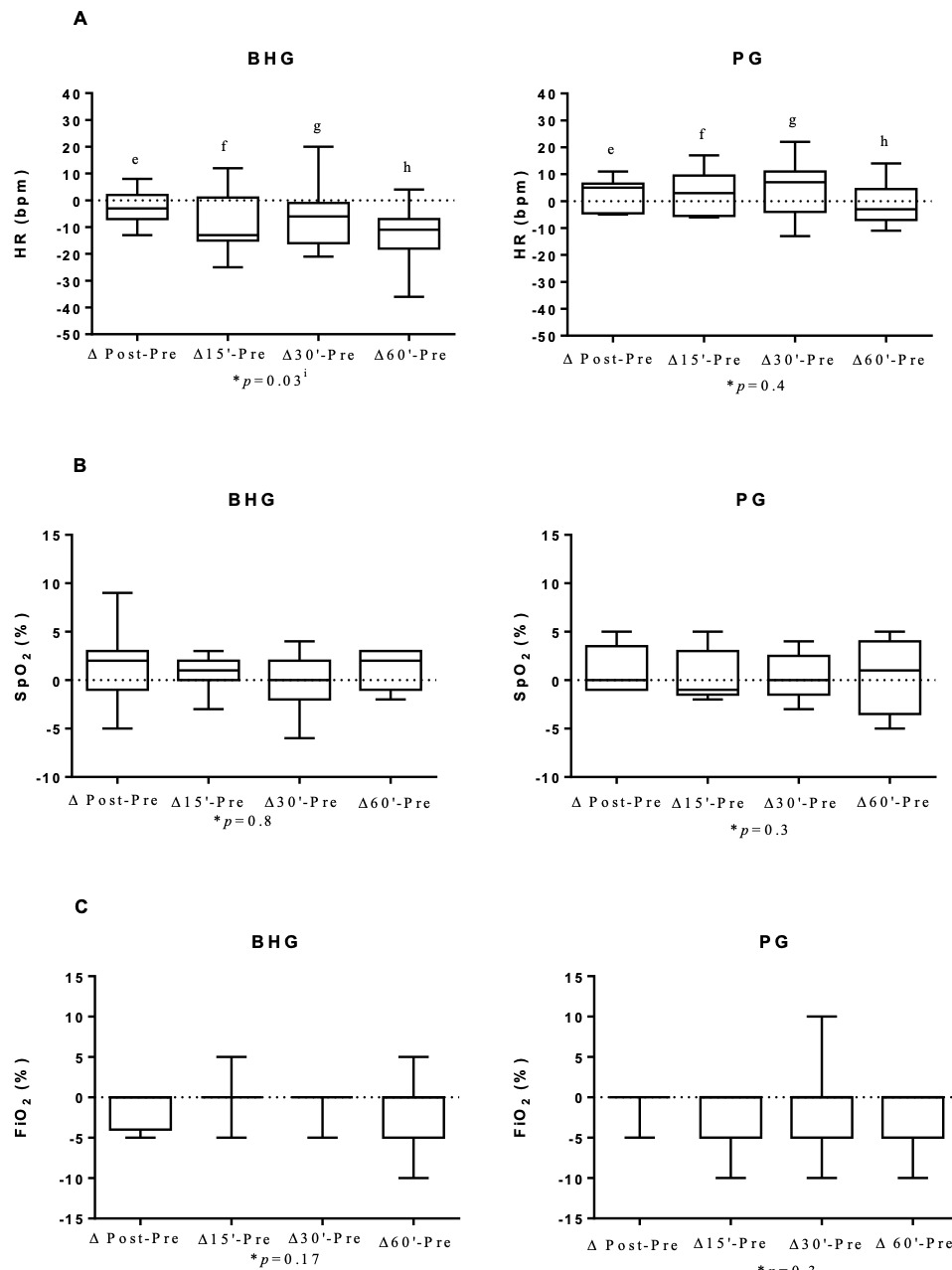
The BHG intragroup comparisons on D6 showed a reduction in HR at 60’ (140[132-153]bpm after bucket hydrotherapy, in comparison to before the intervention (150[145-164]bpm) (p = 0.004). There were no significant differences in any other studied variables on D6.
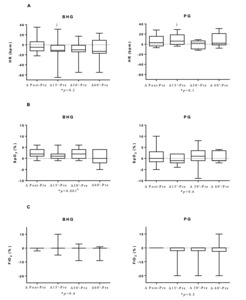
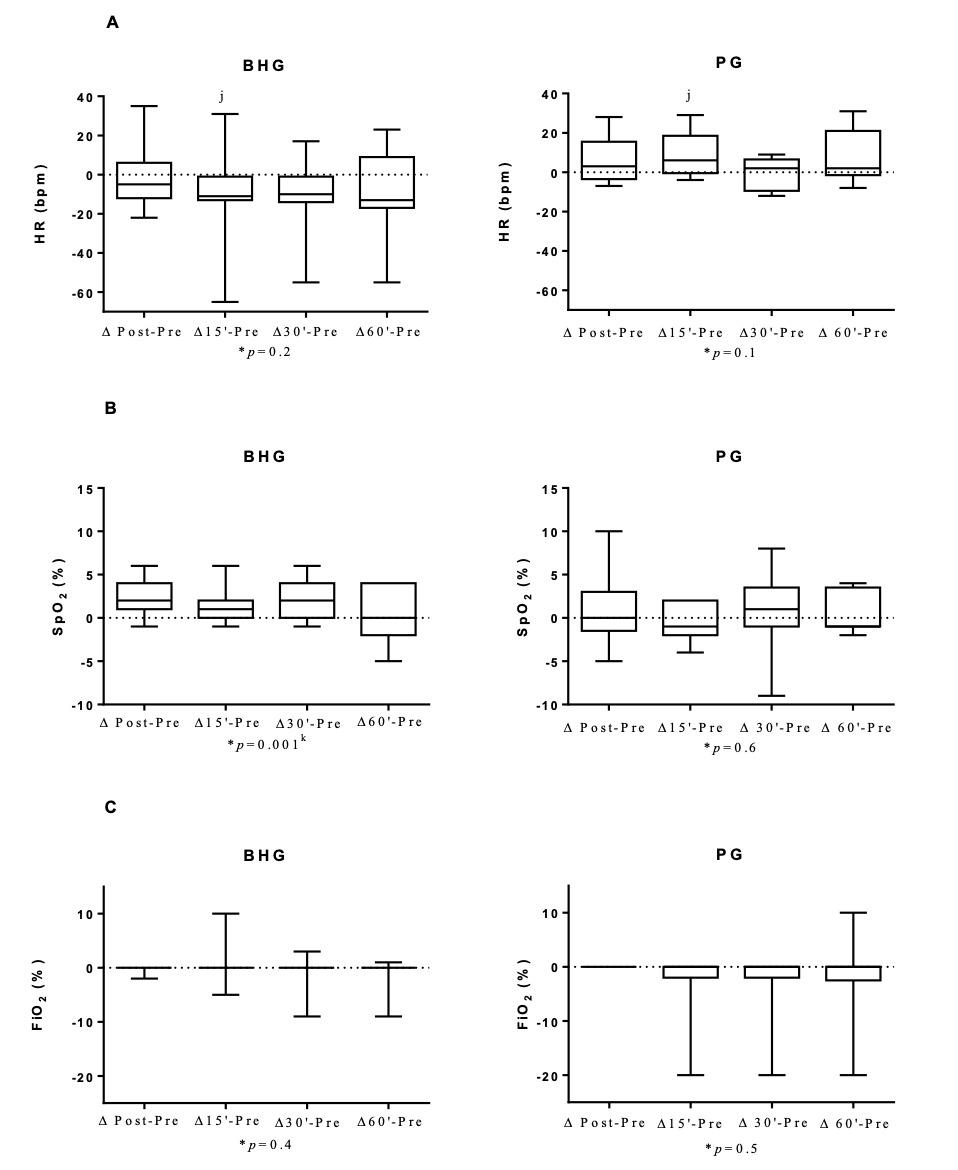
In the BHG, the intragroup comparisons on D12 showed improvements in RR, which was lower at 15’ (42[38-50]breaths/min), 30’ (40[36-47]breaths/min) and 60’ (39[34-51]breaths/min) after bucket hydrotherapy, in comparison to before the intervention (51[45-63]breaths/min) (p < 0.03 for all). SpO2 also improved, with higher values immediately after bucket hydrotherapy (98[97-99]%) in comparison to before the intervention (95[94-98]%) (p = 0.0003). There were no significant differences on any other studied variables on D12.
In the PG, there were no intragroup significant differences in any of the studied variables on D1, D6 and D12.
Changes in all studied outcomes in both groups on D1, D6 and D12 are shown in Figures 3, 4 and 5 and in the Supplementary data. Figure 3 shows that on D1, there was an improvement in ΔSpO2 post - before in the BHG in comparison to the PG (p = 0.03) with a large ES (0.99). Similar positive changes were observed in ΔFiO2 30’- before (p = 0.02) with a medium ES (0.47) and ΔFiO2 60’- before (p = 0.009) with a large ES (0.54) in the BHG in comparison to the PG.
Figure 4 shows that on D6, there was an improvement in ΔHR with a large ES for all analyses, ΔHR post-before (p = 0.04; ES=0.9), ΔHR 15’- before (p = 0.01; ES=1.17), ΔHR 30’-before (p = 0.03; ES=0.99) and ΔHR 60’-before (p = 0.01; ES=1.19) in the BHG in comparison to the PG.
Figure 5 shows that on D12, there was an improvement in ΔHR 15’-before (p = 0.009) with a large ES (0.61) in the BHG in comparison to the PG.
Regarding behavioural parameters, no significant changes were observed in the intra- and intergroup analyses on the evaluated days (D1, D6, and D12). These results are presented in the Supplementary Figures 3, 4, and 5.
Discussion
This study is the first RCT to show the effects of bucket hydrotherapy in preterm infants with BPD. According to the results of the present study, bucket hydrotherapy is a safe approach that can be applied to this population. It also reduces the need for oxygen and improves SpO2 levels with just one session, and lowers HR and RR after cumulative sessions. Bucket hydrotherapy proved to be more effective than conventional physical therapy in reducing FiO2 and improving SpO2 and HR.
Even though improvements in SpO2 have been shown in newborns after bucket hydrotherapy,8,16 none have shown a reduction in FiO2 after the procedure. The present study shows that newborn infants with BPD who received bucket hydrotherapy required less oxygen after the intervention. In the study by Vignochi et al,7 12 newborns with a GA of less than 36 weeks received a single hydrotherapy session, and their SpO2 varied from 91%±3.66% before hydrotherapy to 95.75% ± 3.24% (p = 0.002) after five minutes of intervention. In contrast to these findings, the intragroup comparison in the present study did not reveal any significant difference in SpO2 on the first day of intervention. However, this might be related to the fact that, in the present study, this parameter was used to guide the reduction on FiO2 offered to patients during the evaluation. Whenever the premature infant had a satisfactory SpO2 (above 95%), the evaluator reduced the FiO2 offered. In fact, there was a reduction in FiO2 after bucket hydrotherapy and the intergroup comparison showed that the BHG presented a greater reduction in FiO2, with a large effect size when compared to the PG. These data suggest the importance of bucket hydrotherapy in reducing the need for oxygen in preterm infants with BPD.
On the twelfth day of intervention, when FiO2 was at the lowest setting 21 [21-27]%, infants showed an improvement in SpO2, with values ranging from 95 [94-98]% to 98 [97-99]% after bucket hydrotherapy. Such improvement was only observed in the BHG group. The increase in SpO2 can be explained by the fact that hydrostatic pressure (one of the physical properties of water) triggers changes in respiratory function with an increase in SpO2. The blood is displaced from the lower limbs to the chest region, causing an increase in venolymphatic return, and resulting in an increase in the central volume, which mainly increases the blood flow to the alveoli, which may explain the improvement in gas exchange17 and the consequent decrease in the need for O2 in preterm infants with BPD.
It is also important to highlight the improvements observed in HR after bucket hydrotherapy on D6 and D12, with large effect sizes in comparison to the control group. This finding is in agreement with the study by Sweeney18 that showed a decrease in HR after hydrotherapy, suggesting that this effect is associated with the change to a behavioural state of comfort and relaxation provided by the physical properties of water.18 Hydrotherapy using heated water at thermoneutral temperatures has been shown to reduce levels of stress-related hormones in the body. These responses are hypothetically associated with decreased activation of the sympathetic nervous system, promoting a state of psychophysiological relaxation.1,19 Additionally, floating in water using hydrotherapy reduces the amount of sensory stimuli, which can act to decrease muscle tension. By decreasing the effects of the force of gravity, the buoyancy of the water reduces the activation of joint weight and pressure receptors, thereby mitigating the kinesthetic stimulus and, consequently, the perception of weight-bearing. Thus, the reduction of sensory stimuli that impact musculoskeletal function may have influenced a possible behavioural relaxation in newborns.1,19 The lack of results regarding improvements in HR on D1 of intervention may be because the infants were not yet totally relaxed, since the bucket is a new environment which requires a few sessions for adaptation. The same can be said for RR, which showed a reduction in the BHG only on D12 of intervention.
This improvement in RR is in agreement with the study by Tobinaga et al,8 which evaluated 15 premature newborns with GA=34.2 ±1.66 weeks, assessed before and after hydrotherapy, with values of RR ranging from 55.2 ± 9.16 to 49.3 ±7.9 (p = 0.004) breaths per minute after hydrotherapy. According to the same authors, tachypnea can occur as a result of stress or pain. The reduction in RR observed after bucket hydrotherapy can be associated with a better behavioural state and relaxation provided by the physical properties of water.8
Although the reductions in HR and RR observed were statistically significant, they are not considered clinically relevant due to their small magnitude and the fact that the absolute values remained within normal physiological ranges. However, interpreting the true impact of these changes in premature newborns is challenging, as their physiological and behavioural systems are frequently hyperstimulated in the extrauterine environment compared to the regulated sensory input of the intrauterine setting. Nonetheless, the observed decreases in HR and RR provide supportive evidence that hydrotherapy does not induce stress or pain in this population.8
As for the lack of results regarding the behavior of preterm infants in relation to the sleep and wake phases after hydrotherapy, this study is in accordance with the study by Tedesco et al,9 in which the Brazelton Neonatal Behavioural Assessment Scale was also used in a randomized clinical trial with 34 preterm newborns allocated into an experimental (which received hydrotherapy) or a control (submitted only to diaper change) group. Similarly to the present study, the newborns from the study mentioned earlier9 were also not swaddled for hydrotherapy but were placed naked in the water, allowing them to perform spontaneous movements freely. The authors suggest that the active movements performed by the newborns, as well as the movements facilitated by the therapist during hydrotherapy, may have resulted in a more active behavioural state in the study participants.9
Despite the absence of changes in the behaviour of the studied sample in terms of sleep and wakefulness phases, pain and breathing pattern after hydrotherapy, these variables remained within the normal parameters, which suggests that bucket hydrotherapy is a safe procedure that can be applied in preterm infants with BPD since it does not induce any adverse effects.1
Limitations
This present study has several limitations. First, preterm infants were not categorized by BPD severity or corticosteroid use during the study period. Future studies should include these variables to explore potential differences in intervention response. Second, although multiple assessments (baseline, immediately after, and at 15-, 30-, and 60-minutes post-intervention) were used to maximize the detection of changes, they may have interfered with infant relaxation, potentially affecting some clinical outcomes, which would justify the lack of benefits in other clinical outcomes studied. Additionally, assessments took place up to 60 minutes after the intervention, and in many cases, coincided with the children’s diet schedule, and some of the infants presented crying and irritation related to appetite. Furthermore, the small sample size must also be acknowledged as a limitation. Nonetheless, it is important to emphasize that research in this field is scarce, and conducting studies with this population is particularly challenging due to their vulnerability and clinical complexity. Despite these limitations, this pilot study was an RCT, with both groups (BHG and PG) comparable in terms of PMA, birth weight, sex distribution, BPD severity, weight, and major diagnoses throughout the study, ensuring group homogeneity for comparative analysis. As the first RCT in this area, the findings provide a valuable foundation for future investigations with larger cohorts. Such studies should further explore the short-term physiological and behavioural effects of bucket hydrotherapy, as well as its potential impact on survival and length of hospital stay.
Conclusion
The present pilot study demonstrates that bucket hydrotherapy is a non-pharmacological therapeutic intervention that could contribute to the reduction of HR, RR, and oxygen requirements in preterm infants with BPD, without inducing changes in behavioural parameters.
Contributors
Conceptualisation, Methodology, Validation: DSS, VCE, LSLF ,VSP. Formal analysis: DSS,WSL. Investigation, Resources, Data Curation: DSS, VCE, MLCC. Writing – Original Draft: DSS. Writing – Review and Editing: DSS,VSP, JMF. Visualisation, Supervision, Project Administration, Funding acquisition: VSP. All authors have read and approved the final version of the review.
Financing
The study was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant # 402510/2016-0). This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This RCT was registered prospectively in ClinicalTrials.gov (#NCT03538977).
AI Statement
No generative AI or AI assisted technology was used to generate this manuscript or its content.