Introduction
Aerosol drug delivery is a cornerstone of respiratory therapy, widely utilized in treating a range of pulmonary diseases. However, its effectiveness is often influenced by patient-specific breathing patterns, which can vary significantly depending on underlying respiratory diseases.1 Improving this approach can ultimately enhance treatment outcomes for patients with various respiratory conditions. The pathophysiology of lung disease is reflected in measurements of respiratory mechanics, including compliance and resistance.
Lung diseases are commonly categorized as obstructive and restrictive, each having characteristic lung mechanics. Normal breathing patterns are typically characterized by smooth, consistent airflow, while obstructive lung diseases such as asthma or COPD often result in increased airway resistance, air trapping on exhalation and irregular breathing. Restrictive lung diseases, such as pulmonary fibrosis, lead to reduced lung expansion and reduced lung compliance which may limit tidal volume. In some cases, patients may present with combined obstructive and restrictive patterns, further complicating the mechanics of aerosol deposition in the lungs. Combined obstruction and restriction can also occur, often caused by comorbid pulmonary and non-pulmonary disorders.2 Compliance, a function of pressure and volume, may be greater in obstructive lung diseases than in healthy lungs as elastic recoil is decreased.3 Resistance, a function of airflow, trends greater in obstructive diseases. Both compliance and resistance may influence the delivery of aerosolized medications to patients with obstructive, restrictive, and combined disease conditions.
Despite advances in inhaler technology and drug formulations, the variability in breathing patterns poses significant challenges to optimizing aerosol drug delivery. Only a few studies have investigated clinically relevant parameters associated with disease states and severities,4–9 and have primarily investigated ventilator performance. Little has been reported on differences in inhaled dose using in vitro models simulating these disease-related parameters. Evaluating aerosol drug delivery across different breathing patterns- normal, obstructive, restrictive, and combined- is essential to maximizing treatment effectiveness and refining guidelines and best practices for patients with various pulmonary diseases. Therefore, the purpose of this study is to determine the impact of normal, obstructive, and restrictive breathing patterns on aerosol drug delivery with jet and mesh nebulizers in simulated spontaneously breathing adults. We hypothesized that less aerosols would be inhaled and delivered as obstructive and restrictive breathing became more advanced. Additionally, we anticipated that the use of vibrating mesh nebulizers would improve aerosol delivery in these dysfunctional respiratory mechanics. The research questions of this study are as follows:
-
How does aerosol drug delivery compare among normal, obstructive, and restrictive breathing patterns in simulated spontaneously breathing adults?
-
What is the comparative delivery efficiency of jet and mesh nebulizers in normal, obstructive, and restrictive breathing patterns in simulated spontaneously breathing adults?
Methods
Study Design
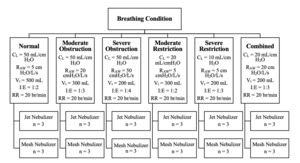
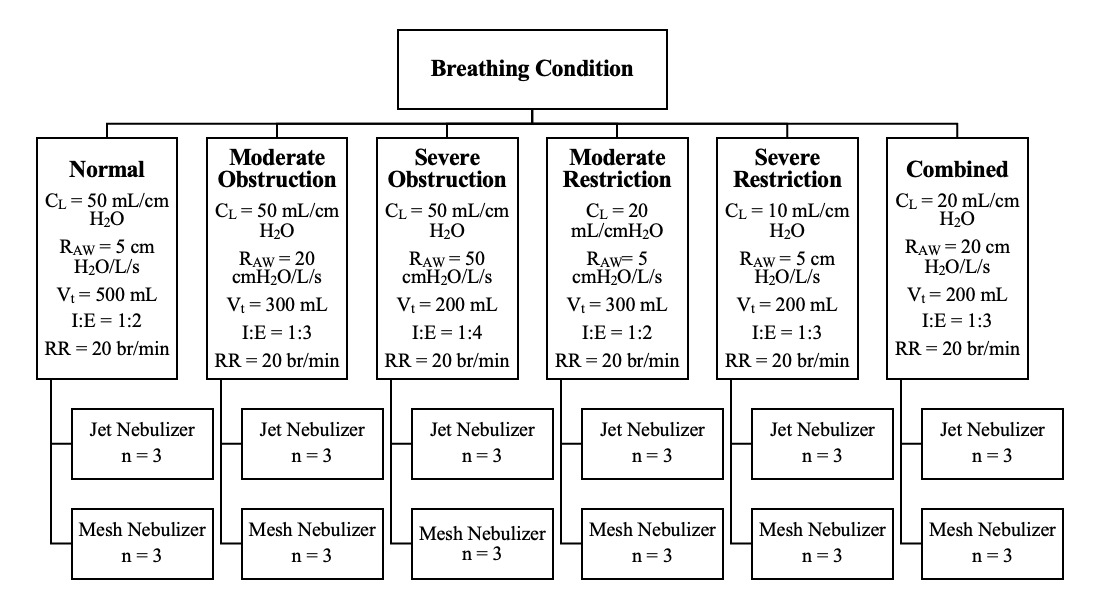
Drug delivery with jet nebulizers (MistyMax 10; CareFusion, San Diego, CA, USA) and vibrating mesh nebulizers (Aerogen Ultra; Aerogen, Galway, Ireland) was compared between six sets of breathing parameters simulating: (1) normal, (2) moderate obstruction, (3) severe obstruction, (4) moderate restriction, (5) severe restriction, and (6) combined (obstruction and restriction) (see Figure 1). A literature review was performed to identify representative breathing parameters for each condition. The QuickLung Breather is restricted to compliance values of 10, 20, and 50 mL/cmH2O and resistance values of 5, 20, and 50 cmH2O/L/s. Based on the selected compliance and resistance values, other operating parameters, including tidal volume, respiratory rate, and the ratio of inspiration to expiration, are also limited. Due to these limitations, modification of the reported values in the literature was required.
Lung Model
A spontaneously breathing adult was simulated using the head and upper airway of a teaching manikin (“Airway Larry” Airway Management Trainer; Nasco Healthcare, Fort Atkinson, WI, USA) connected to a test lung and breathing simulator (QuickLung Breather; IngMar Medical Inc., Pittsburgh, PA, USA). A collecting filter (CareFusion, San Diego, CA, USA) was placed distal to the mainstem bronchi and connected via 22 mm ID tubing to the breathing simulator. A facemask (Adult Elongated Aerosol Mask; Hudson RCI, Durham, NC, USA) was securely attached to the face of the manikin (see Figure 2).
Data Collection
Albuterol sulfate (2.5 mg/3 ml; Nephron Pharmaceuticals, West Columbia, SC, USA) was placed in the nebulizer reservoir. The jet nebulizers (MistyMax 10; CareFusion, San Diego, CA, USA) were operated with an oxygen flow rate of 8 L/min and ran continuously until sputter. A brand-new jet nebulizer was used in each experiment. The mesh nebulizer (Aerogen Ultra; Aerogen, Galway, Ireland) was operated using the Aerogen Pro-X Controller (Aerogen, Galway, Ireland) and connected to oxygen at a flow rate of 2 L/min. The treatment was complete when aerosols were no longer produced. The same mesh nebulizer was used for all experiments. Each experiment was repeated 3 times (n = 3).
The delivered drug was deposited on the collection filter. After each experiment, the filter was removed, labeled, and capped. The drug was eluted from the filter into 10 mL of 0.1 molar hydrochloric acid (J.T. Baker, Radnor Township, PA, USA) with gentle agitation for 3 minutes. The albuterol concentration in the suspension was determined by ultra-violet spectroscopy (Genesys 180 UV-Vis Spectrophotometer; Thermo Scientific, Waltham, MA, USA) at a wavelength of 276 nm.
Data Analysis
The amount of drug deposited on the filter was quantified and expressed as a percentage of the total drug dose placed into the nebulizer. Data was analyzed using GraphPad Prism 10.3 (GraphPad Software, Inc., La Jolla, CA, USA). The deposited dose was compared between the six sets of breathing parameters using the Friedman ANOVA test and uncorrected Dunn’s test. The nominal deposition in each set of breathing parameters was compared between jet and mesh nebulizers using paired t-tests with Holm-Sidak’s multiple comparison test. A p-value of < 0.05 was considered statistically significant.
Results
Effect of Breathing Patterns on Aerosol Deposition
The mean (± SD) percent of the nominal dose of albuterol sulfate deposited for each combination of breathing condition and delivery device is summarized in Table 1.
Aerosol deposition with the jet nebulizer was greater when simulating normal lung mechanics compared to obstructive, restrictive, and combined breathing conditions. The deposited dose was >2.5-fold higher in normal breathing compared to severe obstruction (p = 0.02), severe restriction (p = 0.02), and combined obstruction and restriction (p = 0.001). In contrast, deposited dose in combined breathing was approximately 50% lower compared to both moderate obstruction (p = 0.009) and moderate restriction (p = 0.05). Interestingly, differences in deposited dose were statistically insignificant between moderate and severe obstruction (p = 0.1) and moderate and severe restriction (p = 0.33).
Similar to the jet nebulizer, dose of aerosol deposited with the mesh was greater in normal lung mechanics compared to obstructive, restrictive, and confined breathing conditions. The deposited dose was >2-fold higher with normal breathing than in severe obstruction (p = 0.05), severe restriction (p = 0.001), and combined obstruction and restriction (p = 0.009). There was a 2-fold increase in deposited dose in moderate restriction compared to severe restriction (p = 0.05); however, no significant differences were observed between moderate and severe obstruction.
Comparison of Jet and Mesh Nebulizers on Aerosol Drug Delivery
As shown in Table 1, the delivery efficiency of the mesh nebulizer was significantly greater than the jet nebulizers in all conditions tested. Regardless of breathing condition, mesh nebulizers achieved >2-fold higher deposited dose than jet nebulizers. The largest increases in deposited dose using mesh nebulizers compared to the jet nebulizers were observed in moderate obstruction, severe obstruction, and combined obstructive and restrictive breathing. The deposited dose in moderate obstructive breathing was 4.99% using jet nebulizers and 15.92% using mesh nebulizers (3.2-fold increase; p = 0.0019). In severe obstructive breathing, jet nebulizers delivered 2.86% of administered drugs while mesh nebulizers delivered 8.95% (3-fold increase; p = 0.005). Similarly, mesh nebulizers delivered 7.46% of the administered drug while jet nebulizers only delivered 2.46% (3-fold increase; p = 0.005).
Discussion
Breathing mechanics are widely recognized to directly affect aerosol transport and deposition in the lung. This study has demonstrated the impact of disease-related lung mechanics and breathing patterns on the inhaled dose from continuous aerosol devices. Studies have shown that lung deposition can increase or decrease as much as two times by changing the breathing pattern.10
Our findings suggest that the more advanced the obstruction or restrictive disease, the less aerosol is inhaled and delivered distal to the bronchi with continuous aerosol generation devices. This is independent of disease-related pathology distal to the bronchi, which have been reported to affect the total retention of inhaled aerosol and the distribution of aerosol within the lungs. Recent studies using radiolabeling have demonstrated similar trends in upper respiratory tract deposition when comparing jet and vibrating mesh nebulizers.11–15 Dugernier et al. observed nominal deposition of 17.6% using a vibrating mesh nebulizer and 8.6% when using a jet nebulizer.16 Additionally, they found that the vibrating mesh nebulizer had a higher emitted dose while nearly half of the dose was retained in the jet nebulizer.
For example, lung deposition of inhaled aerosol in patients with obstructive airway disease is closely related with the degree of airways obstruction and can be 2-3 times greater in patients with obstructive airway disease compared to normal.17 From smoking, asthma, small airway diseases, chronic bronchitis and cystic fibrosis, aerosol deposition occurs preferentially at the site of airway obstruction, with an increase of overall pulmonary delivery, altering total and regional deposition.
In contrast, patients with restrictive lung disease show no difference from normal for fine and ultrafine particle deposition fractions. In contrast, restrictive disease has less impact on total lung dose, vs reduced deposition in the less compliant compartments within the lungs.18
This is the first comparison of inhaled dose using an in vitro model simulating the range from normal, moderate and severe, obstructive, restrictive and combined lung disease with continuous aerosol administration. Pulmonary diseases significantly impact the retention and clearance of aerosol particles within the lungs, altering the effectiveness of inhaled therapies. Conditions like chronic obstructive pulmonary disease (COPD), asthma, and pulmonary fibrosis can modify airflow patterns, increase airway resistance, and reduce mucociliary clearance, all of which contribute to prolonged particle retention. In obstructive diseases, airway narrowing traps aerosol particles in the upper and central airways, reducing deep lung deposition. In restrictive diseases, decreased lung compliance limits airflow and alters deposition patterns. These changes affect both therapeutic delivery and particle clearance, impacting treatment outcomes and necessitating targeted approaches for effective aerosol drug delivery.
Our findings suggest that continuous jet nebulization with a mask interface has greater variability in delivered doses across moderate, severe, and combined obstructive and restrictive breathing patterns than the mesh nebulizer. This fourfold higher inhaled dose with normal vs severe disease has not been previously described with either pMDI, DPI or SMI in which the dose is emitted as a bolus that is inhaled in a single breath, resulting in more uniform inhaled dose across the severity of diseases identified.19 Inhaled dose with the mesh nebulizer was more consistent than the jet nebulizer, but still more variability than anticipated with inhalers. It would be worthwhile to increase the sample size in future studies as the deviation between measurements was larger when using the jet nebulizer.
Consequently, a continuous jet nebulizer would not be the aerosol method of choice for treating these conditions. However, some drugs prescribed as aerosol for use in acute care settings are not available as inhalers. Depending on the type of nebulizer used, prescribing clinicians should consider the condition of the patient when prescribing specific doses.
Our findings raise the question of how other types of nebulizers and modes of nebulization such as breath synchronization or small aerosol particles might impact the consistency of aerosol delivery under these tested conditions. Use of small particle breath-enhanced nebulizers have been shown to provide more consistent delivery between normal and subjects with restrictive diseases.20
Limitations
We only studied one model of continuous jet and vibrating mesh nebulizers with one set of adult breathing parameters for each condition. This might not be representative of the range of available nebulizers and individual breathing patterns. While this study effectively compares the aerosol drug delivery efficiency of jet and mesh nebulizers under different breathing patterns, it is important to note that the findings are specific to the particular models of nebulizers used in the study. Therefore, the results should not be generalized to all jet nebulizers, as variations in design and performance among different models may lead to different outcomes. Expanding the sample size and device variety will help ensure more reliable and generalizable results. One limitation of this study is the use of a breathing simulator with fixed compliance and resistance values. This approach may not fully capture the variability seen in human respiratory mechanics, potentially limiting the external validity of the findings. Due to the limitations of this in vitro model, including its inability to fully replicate the complexities of in vivo respiratory physiology and aerosol dynamics, these results may not be predictive of clinical outcomes.
Future Research
This study underscores the importance of comparing different nebulizer types under various breathing conditions. However, to fully understand the nuances of aerosol drug delivery, future research should include a broader range of nebulizer models. Future research with additional nebulizer types, accessories, and interfaces with attention to breathing pattern is warranted. Such comparisons will help identify the most effective devices for different patient needs and ensure that findings are applicable across a wider spectrum of clinical scenarios. Also, understanding where and how much of the aerosolized drug deposits within different regions of the lungs is crucial, particularly in patients with obstructive lung diseases like asthma or chronic obstructive pulmonary disease (COPD). Utilizing imaging techniques such as Magnetic Resonance Imaging (MRI) or Single Photon Emission Computed Tomography (SPECT) can provide detailed visualization of aerosol deposition patterns. For example, MRI has been used to create high-resolution images of the airways and assess the regional distribution of inhaled particles. SPECT imaging, when combined with radiolabeled aerosols, can quantitatively measure the deposition in different lung regions. Therefore, future investigations to better understand regional distribution with measures of physiologic and clinical response are needed. Lastly, this was an exploratory bench study to identify whether changes in selected parameters resulted in discernible differences of inhaled drug distal to the upper airway. Based on our findings, future research should consider using simulators with adjustable parameters to better mimic the diverse range of breathing patterns observed in clinical settings.
Conclusion
In a model of adult spontaneous breathing with continuous jet nebulizer and vibrating mesh nebulizer via a mask, the delivered dose was greatly reduced with parameters representing moderate and severe obstructive, restrictive and combined lung disease compared to normal conditions. The findings of this study emphasize the potential to adjust doses of aerosol therapies when treating patients with severe obstructive, restrictive and combined pulmonary disease. Additional research is required to confirm insights that may guide future recommendations on aerosol therapy in patients with varying respiratory conditions.
Contributors
Dr. Ari conceived of the research idea. All authors contributed to the conception or design of the work, the acquisition, analysis, or interpretation of the data. All authors were involved in drafting and commenting on the paper and have approved the final version.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
All authors have completed the ICMJE uniform disclosure form. Dr. Fink is CSO of Aerogen Pharma. Dr. Ari discloses her relationship with the US Department of Labor, American Association for Respiratory Care, Aerogen Ltd, and Fisher and Paykel Healthcare. Dr. Hoops and Ms. Williams don’t have conflict of interest to disclose with regard to this study.
Ethical approval
Not required for this article type.
AI Statement
The authors confirm no generative AI or AI-assisted technology was used to generate content.

_jet_nebulizer_and_b)_mesh_nebulizer._created_with_b.png)
_jet_nebulizer_and_b)_mesh_nebulizer._created_with_b.png)