Introduction
Pulmonary Embolism (PE) is a potentially fatal yet preventable medical condition characterized by a blockage in one of the pulmonary arteries in the lungs.1 Despite significant advancements in diagnostic techniques and therapeutic interventions, PE remains a significant challenge for healthcare providers due to its high mortality rate, which is estimated to be as high as 30% if left untreated.1 The incidence of PE in Bahrain is not well documented.2 However, it is suggested that the annual rate of Venous Thromboembolism (VTE) in the neighbouring Kingdom of Saudi Arabia (KSA) is 1.7 per 10,000 individuals as of 2022.3,4 This contrasts with the United States, which has a significantly higher prevalence, with an estimated rate of 1-2 VTE cases per 1,000 individuals (0.1-0.2%) each year.5
The clinical presentation of PE is often non-specific, ranging from chest pain and shortness of breath to fainting.1 In some cases, PE can be completely asymptomatic, further complicating the diagnostic process.1 This wide range of symptomatology, coupled with the potentially fatal consequences of missed diagnosis, has led to a high index of suspicion among clinicians and an increase in the use of diagnostic tests for PE. Among the various diagnostic modalities available, Computed Tomography Pulmonary Angiography (CTPA) has emerged as the gold standard choice due to its high sensitivity and specificity.6 However, the increasing reliance on CTPA has raised concerns about its overuse.7 While the prevalence of PE has decreased in recent years, the use of CTPA remains high. This overuse of CTPA not only exposes patients to unnecessary radiation but also places a significant burden on healthcare systems due to inefficient resource allocation.7 In Bahrain, a large cohort study proposes that the PE incidence among clinically suspected patients undergoing CTPA in a tertiary healthcare setting is 11.4%.8 This figure is lower than the recommended positive yield of CTPA set by the Royal College of Radiologists (UK), which advises that the acceptable CTPA positive yield should be between 15.4% and 37%.9
Recognizing these challenges, the National Institute for Health and Care Excellence (NICE) has provided validated guidelines on the diagnostic workup plan for suspected PE cases.10 These guidelines illustrate that if PE is suspected, the 2-level PE Wells score should be used to estimate the clinical probability of PE.10 For people with a likely PE Wells score (more than 4 points), a computed tomography pulmonary angiogram (CTPA) should be offered immediately. A D-dimer test should be offered for those with an unlikely PE Wells score (4 points or less), with the result available within four hours if possible. If the D-dimer test result is elevated, the actions for a likely PE Wells score are to be followed. If it is negative, alternative diagnoses are considered.
Despite these guidelines, there is a lack of adherence among clinicians to NICE guidelines, leading to a lower-than-recommended positive yield of CTPA tests.11 This study aims to assess the positive yield of CTPA orders in suspected PE cases at the Salmaniya Medical Complex (SMC), the largest tertiary hospital in Bahrain, and evaluate the potential reduction in CTPA use achievable through the application of Wells score/D-dimer assessment, as recommended by the NICE guidelines.
Materials and Methods
Study Population
The study population consisted of patients who underwent CTPA at Salmaniya Medical Complex (SMC) from September 1, 2019, to January 31, 2020. The inclusion criteria encompassed any patient aged 18 years or older with a CTPA request registered due to a clinical suspicion of PE. The exclusion criteria were as follows: patients under 18 years of age, patients who did not attend their scheduled CTPA appointments, and those with incomplete or missing records, such as those transferred to the Intensive Care Unit (ICU). Also, any cases where patients underwent CTPA for reasons unrelated to PE were excluded.
Study Design
This was a retrospective study designed to assess the positive yield of CTPA requests in patients with suspected PE and evaluate the potential reduction achievable through the application of Wells score/D-dimer assessment.
Data Collection
Data were collected from the Electronic Patient Records (EPR), which included patient demographics and pre-CTPA workup details, including the Wells score, chest X-ray (CXR), D-dimer, troponin, and brain natriuretic peptide (BNP). Several variables were retrospectively extracted for analysis. These included the patient’s age and gender, presenting diagnosis, baseline and clinical details, as well as laboratory findings such as the D-dimer level and vital signs (systolic blood pressure, diastolic blood pressure, heart rate, respiratory rate, Temperature, SaO2, paO2). The Wells score was calculated for all patients, referencing the patient’s clinical records. The estimated Glomerular Filtration Rate (eGFR) was also calculated using the Modification of Diet in Renal Disease (MDRD) formula published by St George’s University of London.
Data Analysis
For descriptive analysis, the continuous data were represented as means and standard deviations (SD), while the categorical data were denoted as a count (n) and percentage. The yield of CTPA was calculated as the proportion of positive CTPAs out of the total number of CTPA scans conducted. All the results were manually entered into MS Excel 2019 for analysis, and no other statistical analysis tools were utilized.
Ethical Considerations
Ethical approval was granted by the institutional ethical committee at Salmaniya Medical Complex. The confidentiality of the research data was maintained through data coding, where personal identifiers were replaced with unique codes to prevent any direct association with individual participants. Access to the coded data was strictly restricted and limited only to the research team members directly involved in data analysis.
Results
Patient Demographics and PE Diagnosis
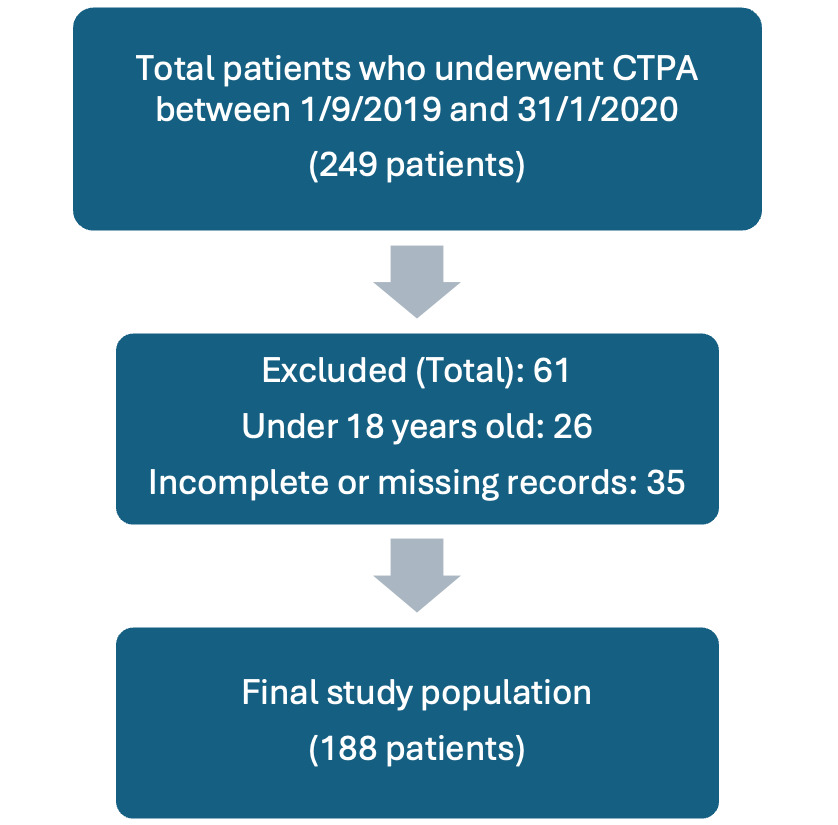
The analysis encompassed 188 patients (mean age: 50 ± 12.3 years, 62.8% female) suspected of having PE. Data were retrieved for all patients who underwent CTPA between September 1, 2019, and January 31, 2020, totalling 249 patients. Of these, 61 patients were excluded based on the previously mentioned criteria: 26 patients were excluded for being under 18 years old, and 35 were excluded due to incomplete or missing records (Figure 1).
Of the 188 patients, only 23 patients (12.2%) were ultimately diagnosed with PE via CTPA. This is significantly lower (p-value < 0.05) than the yield recommended by the Royal College of Radiologists (RCR) (15.4% - 37%). The most common symptoms included dyspnea (57.7%), chest pain (39.7%), heart rate >100 bpm (36.5%), hemoptysis (5.8%), unilateral lower limb pain (15.3%), and unilateral lower limb pain accompanied by edema (12.2%). Common risk factors based on the patient’s histories included recent surgery or immobilization (44.1%), followed by active or past year cancer (8.9%), and pregnancy (4.8%), while previous PE/DVT (4.8%) and estrogen use (0.5%) were relatively less frequent. The demographics and clinical characteristics of the study participants are summarized in Table 1.
Pre-Test PE Probability Assessment and CTPA Findings
Upon calculating the Wells score for our study population, a low-risk Wells score (≤4) was assigned to 129 patients (68.6%), of which only 49 (26.1%) underwent clinical D-dimer testing. Among these, the D-dimer level was elevated in 39 patients. PE was confirmed in 4 out of the 39 patients with low-risk Wells scores and high D-dimers. All 10 patients (5.3%) with low-risk Wells scores and normal D-dimers were PE-negative on CTPA. Eighty patients (47.9%) from the low-Wells score cohort were not followed up with a D-Dimer test. A high-risk Wells score (>4) was assigned to 59 patients (31.4%), of which 19 were diagnosed with PE on CTPA (Table 2).
The most frequent alternative diagnoses identified on CTPA were consolidation (48.9%), followed by pleural effusion (38.3%), atelectasis (23.9%), and ground-glass opacification (22.9%). Less common diagnoses included pulmonary hypertension (6.9%), pulmonary edema (4.8%), diffuse interlobular septal thickening (2.1%), emphysema (1.6%), lung abscess (0.5%), and pneumothorax (0.5%).
Potential Reduction in CTPA Referrals
We focused on two specific patient groups to calculate the potential reduction in CTPA use. The first group, comprising 5.3% of patients, had low-risk Wells scores (indicating PE was unlikely) and normal D-dimers, yet they underwent CTPA. The second group, 42.6% of patients, also had low-risk Wells scores but did not receive D-dimer testing before CTPA. This represents a significant opportunity for improvement, as refining the pre-test probability of PE could potentially avoid unnecessary radiation exposure. Our analysis shows that 5.3% - 47.9% of the total CTPA use could have been avoided using the Wells/D-dimer assessment, highlighting the potential benefit of implementing a more standardized approach incorporating both Wells score and D-dimer assessment as recommended by NICE guidelines.
Discussion
This study aimed to assess the positive yield of CTPA orders in suspected PE cases at the Salmaniya Medical Complex (SMC) and evaluate the potential reduction in CTPA use achievable through the application of Wells score/D-dimer assessment, as recommended by the NICE guidelines. Our findings indicate a gap between current clinical practice and the recommended guidelines, particularly in the use of CTPA for patients with low likelihood of having PE. This highlights a critical area for improvement, as unnecessary CTPA orders reduce the test’s positive yield, expose patients to avoidable radiation and increase healthcare resource utilization. Addressing this gap by implementing guideline-based pre-test probability assessments could significantly optimize diagnostic accuracy and reduce the overuse of CTPA in low-risk patients.
The positive yield of CTPA in our study was 12.2%, lower than the recommended yield by the Royal College of Radiologists (RCR) (15.4% - 37%). This is consistent with a study conducted at Wexham Park Hospital, which reported a positive yield of 16.8%.12 Conversely, Chen et al. (2019) reported a higher yield at a Canadian academic tertiary center (15.9%).11 Sun et al. also reported a 30.7% yield in a single-center experience in Australia, which is much higher than our study.13 These comparisons suggest an overuse of CTPA in our setting, consistent with concerns raised in the literature about the over-reliance on this diagnostic modality.11,12 Furthermore, over the past two decades, several studies have assessed the adherence of CTPA utilization to international diagnostic guidelines.14–16 These studies have predominantly found low adherence rates, which has resulted in an increased use of CTPA scans. A study conducted in New Zealand District Hospital revealed that following international guidelines could have avoided up to 30% of all CTPA scans.14 These guidelines recommend that patients with a low or intermediate clinical probability and a negative D-dimer result can be ruled out for PE without the need for CTPA. On the other hand, CTPA is needed to confirm PE for patients with a low or intermediate clinical probability and a positive D-dimer result. In cases of high clinical probability, CTPA is recommended to confirm PE without the need for D-dimer testing. Similarly, two studies conducted at Tehran’s University of Medical Sciences (2015) and an academic medical center in Philadelphia (2014) showed an adherence rate of 43.9% and 49.5%, respectively.15,16 These findings underscore the need for improved adherence to diagnostic guidelines in the utilization of CTPA scans.12,15
Our study found that the Wells score/D-dimer assessment was not followed in 47.9% of patients (low-risk Wells score with normal/non-tested D-Dimers who had CTPA). This is a significant finding, as adherence to these guidelines could potentially reduce the use of CTPA by between 5.3% and 47.9%. This is in line with a regional clinical audit that found a lower-than-recommended positive yield and suggested that adherence to current guidelines could increase the diagnostic yield and reduce costs and risks associated with CTPA scans.15 Potential barriers to guidelines adherence include diagnostic uncertainty and the fear of missing PE, time pressures due to high patient volumes, knowledge gaps and lack of guideline awareness among clinicians, and institutional or cultural factors (e.g., “better safe than sorry” practices) that may promote a more liberal use of CTPA.15
Several studies have proposed strategies to mitigate the overuse of CTPA scans. For instance, two studies conducted in Germany and the Netherlands recommended integrating an evidence-based clinical decision support (CDS) system into the electronic medical record that provides real-time, patient-specific recommendations to clinicians by flagging certain clinical details and potential concerns about unnecessary testing based on the latest international guidelines, to alleviate the overestimated apprehension or fear of misdiagnosing or missing Pulmonary Embolism (PE). This also ensures that CTPA is only ordered when truly warranted .17,18 Additionally, other studies have emphasized consulting with senior colleagues or other physicians before referring patients for CTPA, which encourages a collaborative approach to patient care, ensuring that CTPA is only utilized in cases where the clinical indications are robust and justifiable. Furthermore, involving senior colleagues can help reinforce clinical judgment and reduce the pressure to order unnecessary tests out of fear of missing a diagnosis or defensive medicine.19
We propose integrating an algorithm-based checklist into the electronic prescription and medication administration software (ePMA) to enhance the management of CTPA referrals. This checklist would incorporate internationally validated assessment tools, such as the Wells and/or Geneva scores, into the process of submitting clinical details required for scan requests. By systematically evaluating the likelihood of PE through these tools, the checklist would guide clinicians in determining whether a CTPA scan is warranted. Furthermore, the system would generate alerts when the pre-test assessment indicates a low probability of PE, prompting reconsideration of the scan request. This approach aims to promote evidence-based decision-making, reduce unnecessary imaging, and ensure that CTPA is utilized appropriately, improving patient care and optimizing healthcare resources.
Our study has a few limitations. It was conducted in a single center; hence, it is important to note that our findings are specific to our study setting. We recommend future multi-center studies to evaluate the applicability of these findings across diverse healthcare environments. Secondly, the Wells score was retrospectively calculated, which may not accurately reflect the clinical judgment at the time of presentation. Additionally, the wide range of potential CTPA reduction (5.3% –47.9%) reflects variability due to incomplete adherence to NICE guidelines, particularly the omission of D-dimer testing in patients with low risk of PE. Despite these limitations, our study provides valuable insights into current diagnostic practices for PE and highlights the potential for improving patient care and resource allocation through adherence to validated guidelines.
Conclusion
In conclusion, despite the huge improvement in PE diagnosis after the introduction of CTPA, there is evidence that this imaging modality is overused globally.16–19 Nevertheless, investigating CTPA utilization in Bahrain is particularly important, as this issue has not been thoroughly examined in the region. Given the potential differences in local healthcare practices and patient demographics compared to global trends, it is also crucial to assess how international guidelines can be effectively adapted locally. Improper CTPA requests are associated with many health-related and financial issues. Algorithm-based clinical guidelines and validated pre-test assessment tools should be applied instantly to guide the rational use of CTPA. Our study underscores the need for improved adherence to recommended guidelines in the diagnostic workup of suspected PE cases. Future research should focus on strategies to improve guideline adherence and evaluate the impact of such interventions on patient outcomes and healthcare resource utilization. We propose integrating an algorithm-based checklist with validated tools like the Wells and Geneva scores into the ePMA system to guide appropriate CTPA referrals, promote evidence-based decision-making, reduce unnecessary imaging, and optimize patient care and resource allocation.
Contributors
All authors contributed to the conception or design of the work, the acquisition, analysis, or interpretation of the data. All authors were involved in drafting and commenting on the paper and have approved the final version. Authors Rabbani Mahmoud Daoud and Ahmed Majeed Mohamed are equal contributors to this work and designated as joint first authors.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
All authors have completed the ICMJE uniform disclosure form and declare no conflict of interest.
Ethical approval
Ethical approval was granted by the institutional ethical committee at Salmaniya Medical Complex.
AI Statement
The authors confirm no generative AI or AI-assisted technology was used to generate content.