INTRODUCTION
Coronavirus disease 2019 (COVID-19) is caused by infection with a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of February 26th, 2024, it has caused over 7 million deaths globally and 18,687 deaths in Croatia.1 The most common cause for hospitalization and intensive care unit (ICU) admission was acute hypoxemic respiratory failure, which has also been the leading cause of death.2,3
Invasive mechanical ventilation (IMV) is often considered the treatment of choice for critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS), particularly in more severe cases.4 However, the optimal strategy, especially for mild forms of ARDS, remains a subject of debate.2,3,5–7 Early intubation strategy used at the start of the pandemic was associated with higher mortality.4,8,9 In certain conditions, such as acute exacerbations of chronic obstructive pulmonary disease, noninvasive respiratory support has been found to improve respiration and reduce the need for intubation, and thereby prevent adverse effects associated with intubation and IMV.3,10,11 However, in patients with acute hypoxemic respiratory failure, conflicting results of the efficacy of noninvasive ventilation (NIV) in preventing intubation and improving survival outcomes have been reported.12–14
Patients typically receive conventional oxygen therapy (e.g. nasal cannula, simple face mask or venturi face mask) until there is a requirement for higher inspiratory flow, increased oxygenation, or to alleviate a high work of breathing.15 Compared to conventional oxygen therapy, respiratory therapy with high-flow nasal cannula (HFNC) and NIV with face mask or helmet have been associated with a decrease in risk of intubation and mortality.16 In case of a mild to moderate acute hypoxemic respiratory failure, HFNC is considered a possible treatment option.10 HFNC respiratory therapy is noninvasive technique of delivering warmed, humidified oxygen with a fraction of inspired oxygen (FiO2) of up to 1.0 and a flow rate up to 60 L/min.15 Arruda et al. emphasize favourable physiological effects of HFNC therapy, which include reducing oxygen dilution and physiological dead space, generating a small positive end-expiratory pressure (PEEP), promoting secretion clearance, and reducing the occurrence of bronchial hyper-responsiveness symptoms.10 With such physiological benefits, HFNC therapy is perceived to be more comfortable for patients and reduces dyspnoea compared to low-flow oxygen therapy.10
Aside from HFNC, noninvasive ventilation (NIV) with continuous positive airway pressure (CPAP) has been described as a potential first-line respiratory therapy for COVID-19 patients.2,17 For patients with an isolated respiratory failure and preserved spontaneous respiratory drive, NIV allows patients to continue breathing spontaneously and avoid endotracheal intubation and sedation.17 Hesitation for both NIV and HFNC use during the COVID-19 pandemic arose from the fear of delaying intubation, the lack of monitoring and control over tidal volume and transpulmonary pressure, as well as possible contamination of the hospital air.18 Furthermore, NIV was avoided due to the fear of developing patient self-inflicted lung injury (P-SILI), but also the lack of familiarity with the device and overcrowded ICUs.18 Reducing and managing aforementioned P-SILI, generated by high respiratory drive, is the biggest concern of spontaneous breathing.17 Jurjević et al. suggest that high PEEP mitigates P-SILI by causing and maintaining recruitment of alveoli which decreases atelectrauma, increases functional residual capacity, and decreases pulmonary oedema.17 Experiments on animal models have shown that high PEEP reduced the risk of P-SILI by reducing the level of spontaneous breathing required and recruiting atelectatic lung areas.19,20
It is still unclear which if any, method of noninvasive respiratory therapy is superior to the other.21 Although the evidence is weak, the Surviving Sepsis Campaign COVID-19 suggests that the HFNC is superior to NIV.22 On the other hand, the Asian Critical Care Clinical Trials Group recommends the use of HFNC and NIV only for patients with mild ARDS, while experts from China also suggest NIV use in moderate ARDS.23–25 The goal of this retrospective study is to compare the efficacy and safety of respiratory therapy with HFNC and NIV with CPAP in patients with COVID-19-related ARDS treated in the COVID ICU at our hospital at the height of the pandemic. In this study, we used the term CPAP to refer to noninvasive ventilation with CPAP mode.
METHODS
This retrospective cohort study utilized data from the COVID ICU at the University Hospital Centre Zagreb between February 2021 and February 2023. Data from electronic and paper health records were retrospectively collected and analyzed.
Ethical Considerations
The study protocol is in accordance with the ethical standards of the Institutional Review Board at UHC Zagreb, as well as with the Helsinki Declaration of 1975. The study was approved under the name “Observational retrospective analysis of CPAP vs HFNC in COVID-19-related acute respiratory distress syndrome” by the Institutional Review Board at UHC Zagreb on September 12, 2022 (document class 8.1-22/156, no. 02/013-JG), with a waiver of informed consent due to the retrospective nature of the study.
Inclusion Criteria
Confirmed COVID-19 diagnosis via RT-PCR; age 18-90 years; acute respiratory distress (determined using the Berlin definition); received HFNC or CPAP during treatment in COVID ICU.26
Exclusion Criteria
Haemodynamic instability (patients in septic shock; hypotension – systolic blood pressure < 90 mmHg or mean arterial pressure < 65 mmHg; need for vasopressor agents); patients who received immediate endotracheal intubation upon admission; patients for whom life-sustaining treatment was withheld; patients who were uncooperative.
Interventions
Continuous Positive Airway Pressure (CPAP) Group
In the CPAP group, patients received NIV with CPAP mode using the standard protocols at UHC Zagreb COVID ICU. Ventilators used were Resmed Astral 150 (manufactured by ResMed in the USA) and The Puritan Bennet 840 (manufactured by Medtronic in the USA). Patients receiving CPAP had to be conscious, adequately sedated, and spontaneously breathing. Before putting on the mask, the procedure was explained to the patient. A suitable mask was chosen with the help of the face gauge included in the package with the mask. Types of masks used were a full-face mask (Nivairo sizes S, M, L, manufactured by Fisher & Paykel Healthcare in New Zealand) or a total face mask (Dimax sizes M, L, XL, manufactured by Sanrai International in Italy). When placing the mask, CPAP and FiO2 were set at 0 cmH2O and 1.0, respectively. All patients were sedated with dexmedetomidine. The CPAP level was gradually raised to a clinical effect at which patient experienced reduced work of breathing. In the first 20 minutes, the CPAP level of 10 cmH2O and FiO2 of 0.6 were reached, and then CPAP was gradually titrated by 1 cmH2O until the breathing effort was minimized.
Patients were intubated in cases of inadequate ventilation (PaCO2 > 50 mmHg), inadequate oxygenation (SpO2 < 88% with FiO2 1.0), persistence of increased work of breathing, or lack of patient adaptation.
De-escalation and termination of CPAP were commenced in the following order. After establishing stable oxygenation, FiO2 was de-escalated to 0.3. After 24 hours spent at 0.3, the CPAP was reduced by 1 cmH2O every 4 hours, and a switch was made from a CPAP level of 5-7 cmH2O to low-flow oxygen through a nasal cannula.
High-Flow Nasal Cannula (HFNC) Group
Patients in this group received HFNC therapy using the standard protocols at UHC Zagreb COVID ICU. The devices used were Fisher & Paykel Airvo 2 (manufactured by Fisher & Paykel Healthcare in New Zealand) and Dräger Hi-Flow Star HFNC (manufactured by Dräger in Germany) - both provide flow rates of up to 60 L/min. Setting up “high-flow” therapy was done in the following order: an adequate size of the nasal cannulas (about 50% of the size of the nostrils) was chosen; the insides of the nostrils was coated with a protective cream; sterile water was connected to the humidifier, and the temperature was set to 35°C, adjusting it according to the patient’s wishes. Initially, the flow was always set to the maximum value of the individual device that the patient could tolerate. FiO2 was titrated according to the value of SpO2, with a recommended FiO2 of up to 0.6.
Decision to intubate was made in case of the following: SpO2 < 88% with the maximum flow (60 lpm) and FiO2 > 0.6 with signs of increased respiratory work (respiratory rate ≥ 30-35/min, use of auxiliary respiratory muscles, subjective feeling of difficulty breathing), or ROX index < 4.88. The FiO2 was then reduced without alteration of the flow until 0.30-0.35 was achieved, while monitoring SpO2 and breathing frequency. Gradual reduction of the flow was then carried out every 6-8 hours by 10 L/min until it reached 30 L/min. When the flow was at 30 L/min, and FiO2 was at 0.3, the transition to low-flow oxygen via nasal cannula was done.
Data regarding HFNC and NIV settings and adjustments were collected from patient health records. The primary outcome was overall intubation rate.
Secondary outcomes included 30-day and 60-day survival rates and frequency of adverse events (e.g., pneumothorax, pneumoperitoneum, and pneumomediastinum).
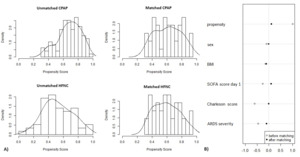
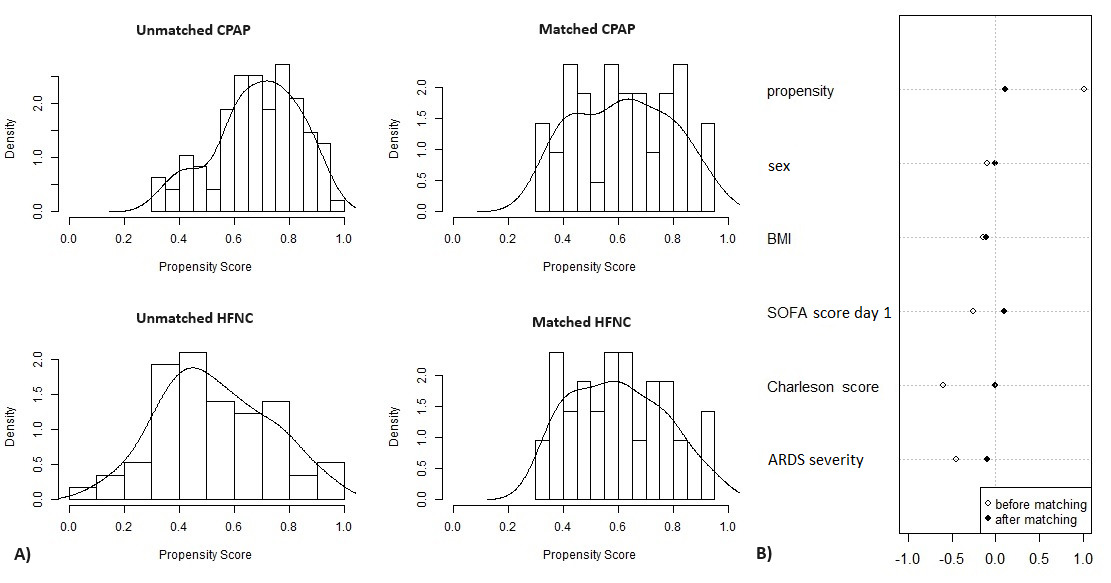
Propensity score-matching analysis
Propensity score-matching analysis was performed in this study with SPSS v 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp) and the matchit package in R software (version 3.3.3 for Windows, Bell Laboratories) and conducted with the 1:1 nearest neighbour matching method, without replacement, with calliper 0.2 (Figure 1). The covariates included sex, body mass index (BMI), sequential organ failure assessment (SOFA) score, Charlson Comorbidity index and ARDS severity. ARDS severity was determined based on PaO2/FiO2 ratio on day 1 of the ICU stay: mild (200 < PaO2/FiO2 < 300), moderate (100 < PaO2/FiO2 < 200), and severe (PaO2/FiO2 < 100). A total of 152 patients – 57 in the HFNC group and 95 in the CPAP group – were matched. The result was 42 patients in the HFNC group and 42 in the CPAP group; 15 HFNC patients and 53 CPAP patients were discarded from analysis either because they were outliers or couldn’t be matched. Overall balance test was x2(df=5) = 0.635, p = 0.986.
Statistical analysis
Categorical variables are described using frequencies and percentages. Continuous variables are summarized as medians (quartiles) or mean values (± SDs) when appropriate. Pearson χ2 test or Fisher’s exact test was used to determine the association between categorical variables. The Student’s t-test or the Mann-Whitney U test was used for continuous data appropriately. Log Rank test and Kaplan-Meier curve were used for survival analysis. Analysis of variance (ANOVA) or the Kruskal–Wallis test were used for comparisons between more than two groups. Statistical analysis was performed using SPSS, version 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). A p-value of < 0.05 was considered statistically significant, and power was 0.981.
RESULTS
719 COVID-19 patients aged over 18 years with acute respiratory distress treated in COVID ICU in UHC Zagreb met study criteria based on electronic health record data. Out of the 719 patients, 176 were treated with HFNC and 132 with CPAP. After elimination of incomplete data, 57 HFNC and 95 CPAP patients remained. After propensity score matching, there were a total of 84 patients included in this study: 42 received HFNC, and 42 CPAP (Figure 2).
Baseline patient characteristics were similar between the groups (Table 1). The mean age of patients was 65.62 ± 12.75 y (range 35-87 y) and 68.40 ± 10.61 y (range 29-87 y) in the HFNC and CPAP group, respectively. At the time of the admission to the ICU, fifty (59.5%) patients had severe ARDS. Stratification of patients based on ARDS severity did not differ significantly between the HFNC and CPAP groups (p = 0.473). All patients received treatment in the ICU with remdesivir and dexamethasone. PaO2 at the time of admission (p = 0.907) and PaO2/FiO2 ratio on day 1 of the ICU stay (p = 0.247) were similar between the two groups.
On day 1 of the admission to the ICU, the HFNC group’s oxygen flow was set at a mean value of 54.29 ± 1.84 L/min with an average FiO2 of 0.73 ± 0.18%. For the CPAP group, mean PEEP value set on day 1 was 13.90 ± 2.85 cmH2O, within the institute’s protocol recommended range, and with an average FiO2 of 0.55 ± 0.14%.
Primary outcome
The intubation rate in the first 7 days of the ICU stay was 61.9% (26/42) and 16.7% (7/42) in the HFNC and CPAP groups, respectively (p < 0.001) (Table 2). Patients in the HFNC group were shown to have a higher probability of intubation during the first 7 days in the ICU compared to the CPAP group (OR 8.125; 95% CI 2.921-22.598). The total intubation rate during patients’ ICU stay also differed significantly between the two groups (p = 0.004); 71.4% (30/42) patients required a switch to intubation in HFNC group and 40.5% (17/42) in CPAP group. Patients in the HFNC group had higher overall probability of intubation than CPAP group (OR 3.676; CI 1.480-9.232). Analysis of cumulative incidence of intubation revealed increased need for intubation in the HFNC group (p = 0.003); see Figure 3a.
Secondary outcomes
Table 2 lists the secondary outcome results. The 30-day and 60-day survival rate was 47.6% (20/42) and 35.7% (15/42) respectively in HFNC group and 66.7% (28/42) and 54.8% (23/42) in CPAP group. The survival analysis for the 30-day and 60-day cumulative mortality has shown a marginally significant increase in cumulative risk of death in the HFNC group (p = 0.050 and p = 0.043 as compared to the CPAP group); see Figure 3b.
Pneumothorax, pneumomediastinum, and subcutaneous emphysema were observed as possible complications of treatment in both groups. A higher incidence of pneumomediastinum was observed in the CPAP group (10/42) compared to the HFNC (3/42) (p = 0.039; OR 3.958; 95%CI 1.003-15.628). However, no difference in mortality was observed in patients who developed pneumomediastinum (p = 0.697). The incidence of subcutaneous emphysema or pneumothorax did not differ significantly between the two groups. No difference was observed between the HFNC and CPAP groups in time to onset of any of the three complications.
Change in Observed Respiratory Parameters
Following initiation of both modalities, a significant improvement in PaO2 values was observed in the CPAP group compared to the HFNC group on day 1 (p = 0.004) and later on day 3 (p < 0.001) of the ICU stay (Table 3). An increase in PaO2 on day 1 compared to values observed prior to the admission to the ICU was significantly higher in the CPAP group (+25.43 ± 24.45 mmHg on average) compared to the HFNC group (+9.15 ± 15.53 kPa on average) (p = 0.002). By day 3, improvement in PaO2/FiO2 ratio was significantly higher in the CPAP group (p < 0.001); average changes of -16.90 ± 54.33 and +85.40 ± 82.34 were observed in the HFNC and CPAP groups respectively (p < 0.001).
DISCUSSION
Our study aimed to compare the efficacy and safety of respiratory therapy with HFNC and NIV with CPAP in patients with COVID-19-related ARDS. A higher probability of intubation was observed in the group of patients who received respiratory support by HFNC compared to patients who received CPAP (p = 0.004). During the first week in the ICU, the HFNC group was shown to have 8 times higher probability of intubation compared to the CPAP group (p < 0.001). Marginally significant difference favouring CPAP over HFNC was observed at 30 days (p = 0.050) and was more pronounced at 60 days (p = 0.043). Patients in the CPAP group had shown a significantly better improvement in PaO2 following initiation of noninvasive ventilation (p = 0.002) as well as PaO2/FiO2 ratio by day 3 of the ICU stay (p < 0.001).
Al Hashim et al. have previously compared efficacy between noninvasive ventilation with face mask, helmet, and HFNC in patients with COVID-19-related respiratory failure but found no significant difference in intubation rate or survival between the three methods.2 Their study had a similar sample size to ours (52 patients in CPAP group, 52 in the helmet CPAP group, and 47 patients in the HFNC group). A lack of difference in intubation rates between the two modes was also reported by Duan et al. and Arabi et al.; however, unlike this study, both studies used NIV with pressure support.21,27
Unlike our study, the majority of available research on the topic investigates and compares the use of NIV with pressure support in COVID-19-related ARDS, making the comparison of results difficult. For example, a randomized clinical trial by Grieco et al., which compared HFNC to helmet CPAP followed by HFNC, reported significant difference in intubation rate between the two groups (51% vs 30%; p = 0.030), which is in line with our results.28 However, no difference was present in number of days free of respiratory support within 28 days. Frat et al. reported lower 90-day mortality rate in HFNC group but no difference in intubation rates between HFNC and NIV.29 It should be noted, however, that in the study by Frat et al., similar PEEP levels were applied in both groups, unlike our study where patients in CPAP group were treated with significantly higher CPAP levels. Also, Frat et al. utilized higher tidal volumes (9.2 ± 3.0 mL/kg on average, which is above recommended values).8 The favourable effect of high CPAP levels used in our study corroborates the findings of Jurjević et al. and matches the positive outcomes of high PEEP demonstrated on experimental animal models by Morais et al.17,19
Strengths and Limitations of the Study
The main limitation of this study is its retrospective nature which narrowed down sample size as most patients had incomplete data set.
We did use propensity score matching to eliminate the influence of main confounders (e.g., sex, BMI, SOFA score, Charlson Comorbidity index, ARDS severity), and we strongly believe that despite relatively small sample size, our results are representative.
CONCLUSION
In conclusion, our results suggest that initiating CPAP has benefits in treatment of ICU patients with COVID-19-related ARDS when compared to HFNC. Patients in the CPAP group had a significantly lower incidence of intubation than patients treated with HFNC, and have also shown a significant improvement in 60-day survival. This study demonstrates that there is room for CPAP in treating COVID-19-related ARDS; however, further research is necessary. For example, prospective studies about this topic are needed, as well as further investigation into safety limits of applied PEEP and FiO2 levels.
Contributions
Conceptualization: Ivan Šitum (Lead), Anđela Babić (Supporting), Daniel Lovrić (Equal). Formal Analysis: Ivan Šitum (Lead). Methodology: Ivan Šitum (Equal), Ante Erceg (Supporting), Anja Mandarić (Supporting), Dora Karmelić (Supporting), Gloria Mamić (Equal), Nikolina Džaja (Supporting), Mirabel Mažar (Supporting), Daniel Lovrić (Equal). Project administration: Ivan Šitum (Lead), Anđela Babić (Equal), Slobodan Mihaljević (Equal), Mirabel Mažar (Supporting), Daniel Lovrić (Equal). Writing – review & editing: Ivan Šitum (Equal), Lovro Hrvoić (Equal), Ante Erceg (Supporting), Anja Mandarić (Supporting), Dora Karmelić (Supporting), Gloria Mamić (Supporting), Nikolina Džaja (Supporting), Anđela Babić (Supporting), Slobodan Mihaljević (Supporting), Mirabel Mažar (Supporting), Daniel Lovrić (Supporting). Investigation: Lovro Hrvoić (Supporting), Gloria Mamić (Supporting), Nikolina Džaja (Equal). Visualization: Lovro Hrvoić (Equal). Supervision: Lovro Hrvoić (Supporting), Slobodan Mihaljević (Equal). Writing – original draft: Lovro Hrvoić (Lead), Gloria Mamić (Supporting), Nikolina Džaja (Equal). Data curation: Ante Erceg (Equal), Anja Mandarić (Supporting), Dora Karmelić (Equal), Anđela Babić (Supporting). Resources: Slobodan Mihaljević (Lead).
Funding
No funding was received for conducting this study or for the preparation of the manuscript.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose. The authors declare no conflict of interest.
Ethics Approval
The study was approved by the Institutional Review Board at UHC Zagreb on September 12, 2022 (document class 8.1-22/156, no. 02/013-JG), with a waiver of informed consent due to the retrospective nature of the study.
AI Statement
The authors confirm no generative AI or AI-assisted technology was used to generate content.


**_kaplan-meier_plot_of_the_cumulative_need_for_intubation_from_admission_to_the_icu_.png)

**_kaplan-meier_plot_of_the_cumulative_need_for_intubation_from_admission_to_the_icu_.png)