Introduction
Volatile agents like sevoflurane and isoflurane are increasingly used for sedation in adult critical care since the availability of miniature vapourizing devices that are compatible with dual-limb critical care ventilators.1,2 The standard position for the SedaConDa® Anesthetic Conserving Device (ACD®)(Sedana Medical, Sweden) is between the patient’s endotracheal tube and the wye of the ventilator circuit. In this configuration, the device delivers the anesthetic vapour, functions as a Heat and Moisture Exchanger (HME) and has the additional benefit of reflecting the volatile agent, trapping approximately 90% of exhaled volatile agent and recycling back to the patient.3 The potential drawback of this positioning is the added dead space to the patient’s breathing circuit. The smallest version (ACD®-S) used with this configuration requires the patient to have tidal volumes of greater than 200mL4 to ensure appropriate ventilation in the setting of the increased dead space and, therefore, has faced limited use in infants and children with tidal volumes under 200 mL.
An alternative position that places the ACD®-S on the inspiratory outlet of the ventilator has permitted the use of volatile gases for sedation in infants and children with tidal volumes between 30- 200mL.5,6 This position eliminates any additional dead space to the ventilator circuit, but the ACD no longer recycles volatile agent exhaled by the patient and does not provide effective humidification. In this context, it is anticipated that higher infusion rates of the anesthetic may be required in addition to the use of active humidification. The addition of ventilator circuit humidification could also impact the capacity of passive scavenging systems as they are indiscriminate in their absorption of volatile agent and water vapour. While the SedaConDa® system recommends more frequent changes for their commercially available charcoal-based (FlurAbsorb) scavenging canisters,7 the capacity for other scavenging systems to manage water vapour is unknown. Deltasorb® (Blue Zone Technologies, Canada), a volatile capture technology that utilizes silica zeolite for volatile capture as opposed to the traditional activated charcoal,8,9 has previously demonstrated as having excellent volatile capture and scavenging with the ACD® using the standard circuit positioning and without active humidity.10,11 Some centres have adopted this scavenging system as it aligns organizationally with environmental initiatives to reduce waste anesthetic gases emissions (known to be a potent greenhouse gas)9,12,13 through a proprietary system to extract scavenged volatile agent from their absorbant, reprocess and rebottle the volatile agent in liquid form for future use in patients.
Use of scavenging systems with inhaled volatiles is essential to ensure the safety of the health care workers.14,15 In a bench study, our primary aim is to to evaluate the efficiency of volatile gas scavenging and resultant occupational exposure of SedaConDa ACD®-S when located on the inspiratory limb with active humidication in paediatric-sized patients. The effectiveness of scavenging will be measured by calculating environmental volatile exposure in a typical paediatric intensive care environment as well as the percentage of gas scavenged. The second aim is to estimate the scavenging canisters required frequency of replacement by assessing the volume of water vapour and isoflurane captured by the scavenge system.
Approach (Methods)
This study was reviewed by The Hospital for Sick Children IRB and deemed to meet the criteria for exemption.
In a four-bed PICU room at a quaternary level children’s hospital, four ventilators were set at each bed spot simulating two children (10-year-old, 30 kg) and two infant (6 month-old, 5 kg) patients.
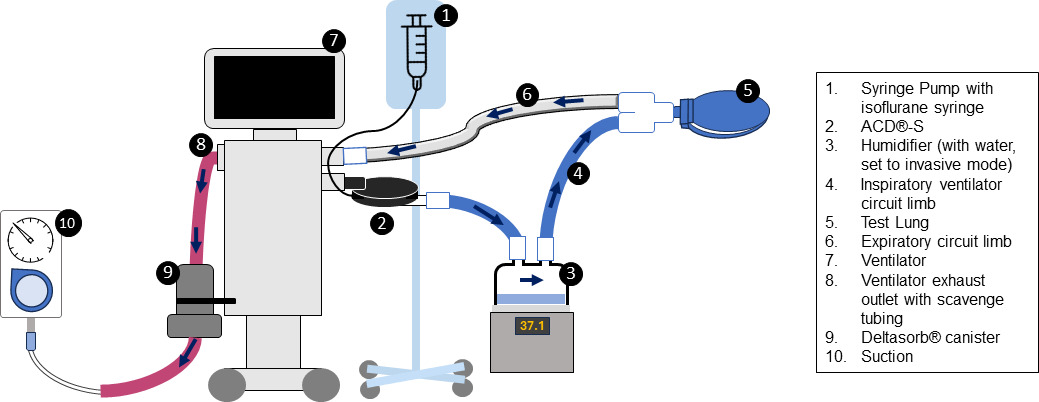
The configuration used in the bench study is shown in Figure 1. Two Servo-i® and Servo-U® ventilators (Getinge, Sweden) were set up with active humidification using Fisher-Paykel MR850 humidifiers and the RT266 and RT 380 Evaqua2™ circuits (Fisher-Paykel, New Zealand). Humidification was set to invasive ventilation, which uses automatic algorithms targeting 37°C (44 mg/L, body temperature and pressure, saturated), achieving this following a brief warming up period. No interruptions to the ventilation or collection (no ventilator or scavenging disconnects) occurred during testing. All four ventilators had a single Deltasorb® Series 1000 Anesthetic Collection Device (Blue Zone Technologies, Canada) (the “canister”) attached to the expiratory outlet (port), with active scavenging attached to the outlet on the canister with standard wall suction set to -20mmHg similar to prior studies [10]. Two of the ventilators had the SedaConDa® system (ACD®-S and SedaConDa® Syringe) positioned on the inspiratory limb; with a BD Alaris PC Unit and Syringe Module (Alaris Medical, USA) running isoflurane (3 mL/hr). The ventilators were set to typical institutional settings to clinically represent the size of an infant and larger child. For the infant representation, we used: paediatric category, the RT266 circuit, respiratory rate of 30 breaths/min, pressure control 15 cmH2O above positive-end expiratory pressure (PEEP) 5cmH2O; inspiratory time 0.5s and FiO2 0.40, achieving a measured tidal volume of approximately 15-20 mL and minute volume of 0.4 LPM. For the child representation, we used the following settings: adult category, the RT380 circuit, pressure-regulated volume control, respiratory rate of 25 breath/min, set tidal volume 200mL, inspiratory time 0.8s, with PEEP 5 cmH2O and FiO2 0.4 achieving a minute volume of 5.2 LPM. Two sizes of simulated test lungs were used for the infant and child setup, respectively.
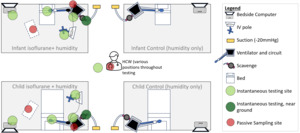
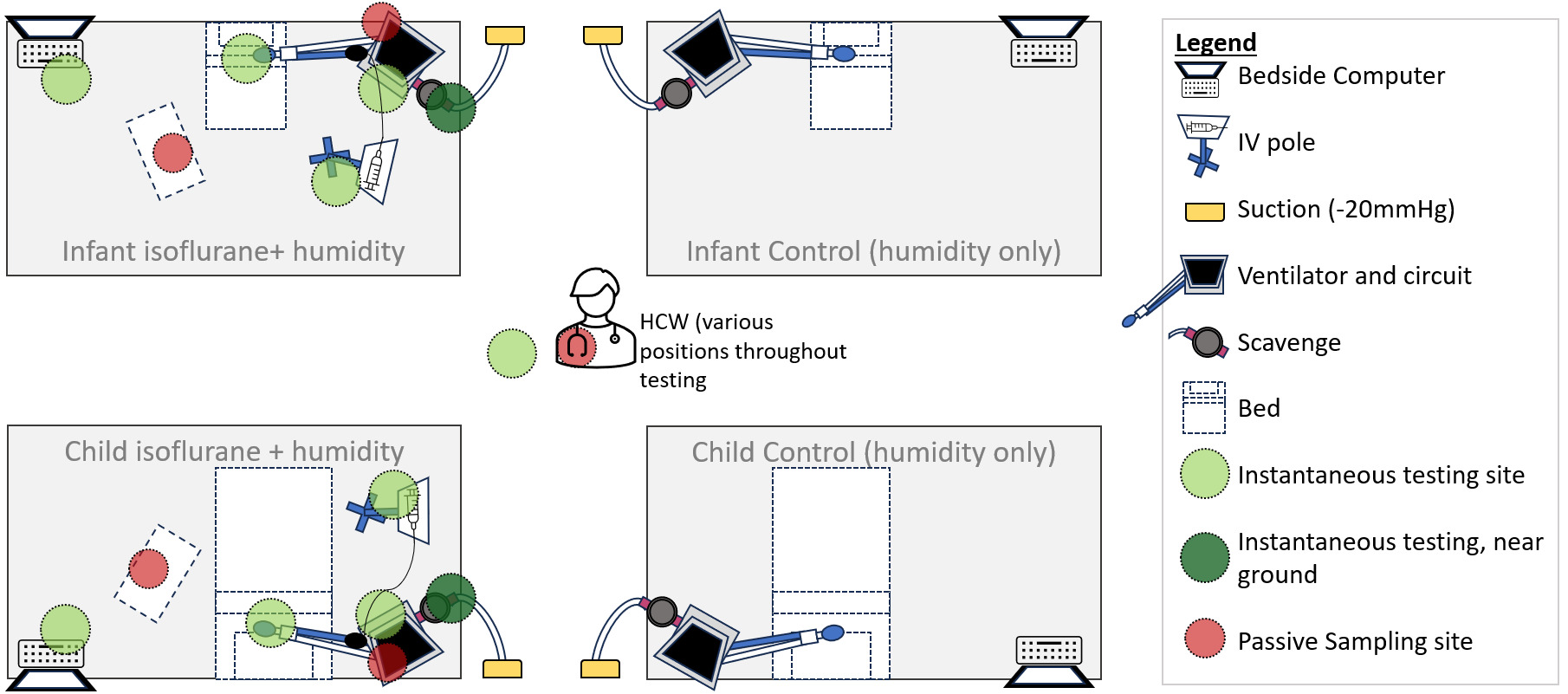
For passive sampling, four diffusive 574 Anesthetic Gases Monitor badges (Assay Technology, USA) were opened at the start of testing and placed at points around the clinical environment where clinicians may typically stand for longer periods. Passive sampling involves using coin-sized badges that capture isoflurane from the environment in an activated molecular sieve, allowing characterization of the average exposure over the testing period. Four sampling locations were used - two attached directly to the ventilator, and two located at the bedside/workstation for both the infant and child ventilator simulations (Figure 2). A fifth badge was attached to the collar of the author (JM), who moved around the room during the day, simulating a worker’s typical work routine. The badges were closed upon collection, sealed in the designated return pouch, and shipped to Assay Technology Laboratory (California, United States of America) for analysis. The lab utilizes toluene desorption and headspace analysis by gas chromatography with flame-ionization detector to determine the time-weighted average exposure (TWAE) concentration of isoflurane.16
Instantaneous measurements for isoflurane room concentrations were measured hourly using a MIRAN SapphIRe Portable Ambient Analyzer (Thermo Scientific, USA) at six points in the room - at the bedside computer, at ventilator circuit, at the infusion pump, at the test lung, near the scavenging exhaust area and the centre of the room (Figure 2). The analyzer contains a single-beam infrared spectrophotometer to specifically and accurately detect isoflurane in parts per million.17 The air exchanges are reported by the hospitals’ facilities operations and exceed eight exchanges per hour. Following the end of testing, the canisters were capped (both ends), serial numbers recorded with testing details and returned to Blue Zone Technologies for analysis. During the testing, the total infused isoflurane was estimated to be 29.25 grams (infusion rate 3mL/hr x 6.5hr x density of isoflurane 1.5g/mL). Amount of water administered was calculated using water vapour level, minute ventilation and total run time of the experiment (water vapour level [44mg/L based on 100% humidity] X MV X 6.5hr). Using their proprietary process of desorption to extract both water and isoflurane from the canister, Blue Zone recorded the estimated weights of extraction from each scavenging canister for isoflurane and water that was captured during testing and provided a report to the authors. Of note, the extracted volumes are estimates of the water and isoflurane during testing that was captured by the scavenge (known to be attributed to either water or volatile agent in the desorption process) performed by Blue Zone Technologies and were not verified by the authors.
Environmental volatile gas exposure is presented as parts per million (ppm), and is compared to the standards set about Ontario TWAE and Ceiling Limits from O. Reg. 833,18 which sets the ceiling limits of exposure at 10ppm and the time-weighted average exposure (TWAE) at greater than 2ppm. Exposure is presented as either instantaneous measures or as an accumulated exposure over the 6.5-hour experiment.
Findings
Table 1 summarizes the instantaneous measurements and continuous sampling results for isoflurane. All detected levels were below the Ontario occupational TWAE limit of > 2ppm and the ceiling limit of > 10ppm. The instantaneous point sampling exposure ranged from 0 to 0.6ppm, with the latter being detected beside the endotracheal tube/airway. The continuous sampling, which detected total isoflurane exposure during the 6.5-hour experiment was 0.1ppm for both the infant and child experimental configurations at both the monitored bedspaces and the ventilator screens. The isoflurane exposure detected on a clinician working bedside during the 6.5-hour experiment was 0.1ppm. The results demonstrate minimal ambient concentrations of isoflurane detected while the scavenging system was active.
Deltasorb® Series 1000 desorption demonstrated 27/29.3 grams (92.3%) of isoflurane and 6/6.9 grams (87%) of water was collected during the 6.5-hour infant (estimated 0.4 LPM) test, whereas 18/29.3 grams (61.5%) of isoflurane and 40/89.2 grams (44.8%) of water were collected during the 6.5-hour (estimated 5.2 LPM) child simulation (data not shown). Based on child simulation with our testing conditions (ventilator), and the capacity reported by BlueZone, our collection suggests that changes should be performed at an interval of two days with the goal of changing the canister prior to reaching capacity. We recommend the two day interval with the expectation that the canister will have reached 70% of total capacity, thereby providing a small buffer.
Discussion
This bench study is the first to evaluate the occupational isoflurane exposure to bedside clinicians and hospital staff providing routine care of a paediatric patient treated with volatile anesthetic agents using a miniature vapourizing device in the setting of active scavenging system with device placement on the inspiratory limb. The findings show that Deltasorb® volatile capture scavenging is effective at maintaining safe atmospheric volatile levels in the setting of large volumes of water vapour produced by active humidification in a closed (leak-free) ventilator system. Strengths of our study include testing environmental exposure in multiple locations using two methods of instantaneous point sampling and continuous sampling over 6.5 hours. The multiple locations sampled represent areas where specialized healthcare workers (respiratory therapists, nurses, clinicians, physical therapists) frequent during routine patient care. All exposure values were well below Canadian and international regulatory limits.12,18 The Deltasorb® data demonstrated desorption and extraction of both large volumes of water and isoflurane which supports that these systems effectively absorb isoflurane in the presence of active humidification and maintain a safe working environment.
Of particular importance, minute volume had an impact on canister duration and capacity. In the infant model represented with a low minute volume (0.4LPM), the single canister absorption was very effective, absorbing nearly all isoflurane and water vapour. In contrast, a single canister failed to capture the entirety of the administered water vapour and isoflurane in the larger child model with higher minute volumes (5.2LPM). These results are consistent with the described limitations of volatile capture technology at higher volatile flow rates or higher volatile concentrations.9,19 Despite this, volatile exposure remained well below occupational health standards in both models, suggesting that the addition of active scavenge (by adding suction) is effective in clearing the volatile agent should the volatile capture system have some degree of ineffectiveness. We chose to evaluate a single canister in paediatrics, rather than the tandem approach of two canisters used in previous studies.10 Our findings of incomplete volatile capture with higher minute volumes are potentially due to gas flow through the canister exceeding the necessary dwell time for complete absorption of isoflurane8,19; therefore, we would recommend using two canisters as previously reported for any larger-sized paediatric patient clinically.10,11 Clinicians may choose to adopt a standardized approach for all patients for simplicity or may adopt a standardized approach for smaller and larger patients, determined potentially by a minute volume threshold.
An essential component of respiratory care in neonatal and paediatric critical care is use of active humidification20 rather than routine use of HMEs. The importance of minimizing dead space, avoiding any added resistance, and optimizing secretion clearance are important components of respiratory management and are culturally engrained practices in PICU. This has ultimately made HME adoption relatively limited in the paediatric setting. Due to the instrumental dead space issue, some adult studies have raised the possibility of using the ACD®-S with its dead space of 50 mL in the majority of adult patients in respiratory failure who require lung protective ventilation strategies.2 If applying these same precautions, this would result in the ventilator inspiratory limb positioning of the ACD®-S for most paediatric cases. Understanding practice differences between adult and paediatric patients, and the implications on scavenging system performance with active humidification is imperative to ensure the safety of all PICU providers.
Our bench test had several limitations. Firstly, it was performed under very controlled conditions for reporting. It is possible that in daily care, there are variabilities in the ventilation settings and patterns, isoflurane infusion rates, circuit disconnections (potentially required for open suctioning, desaturation requiring emergency bagging, or accidental disconnection) and the use of uncuffed endotracheal tubes that may cause higher transient ambient isoflurane exposure to bedside clinicians in the pediatric environment. However, given the lower doses of isoflurane used in ICU settings, low exposure measurements in our study and the presence of room air exchanges, we expect that ambient isoflurane levels would remain safe with the scavenging system tested. Second, our study recorded slightly lower tidal volumes with the infant model than the suggested limit for clinical use (15-20mL); this was a limit of the infant test lung that was available and a pragmatic decision to proceed with testing by the authors. Third, our test conditions used a specific ventilator with low bias flow. It is possible that other ventilators would create different results, and we would caution extrapolation of these results without considerations to differences in the ventilation conditions. Various factors can contribute to both the scavenging effectiveness in the presence of active humidity - the delivered dose of isoflurane, minute volume, ventilator bias flow, efficiency of the humidifier, and condensation in the circuit all can impact the volume of water vapour entering the scavenge system. Finally, we ended our experiment prior to the canister being fully saturated and therefore we utilized the extraction data to estimate the timing for canister exchange, suggesting a canister change at 70% capacity.
The awareness of volatile agents and potential therapeutic benefits in paediatrics is well described.21,22 Historically, the most common indication for the use of volatile gases in paediatric critical care has been to treat bronchospasm in children with severe status asthmaticus that is refractory to conventional therapies.22–24 It has also been utilized in children who are challenging to sedate5,6 and as a treatment for refractory status epilepticus.25 While the use of volatile agents in paediatrics has existed for many years, the widespread adoption or availability of this therapy in the critical care environment has been limited by equipment and trained operators.21,22 The availability of miniature vapourizing devices that are compatible with ICU ventilators has sparked a resurgence of clinical interest in this therapy. The use of these devices remains limited in paediatrics, with limited practice guidance on scavenging safety, given the specific needs of placing the device on the the ventilator inspiratory limb with need for higher infusion rate of volatile agents and active humidification. Our study adds to the growing body of literature5,6 on the safety of using miniature vapourizing devices and inhalational volatile based sedation in critically ill paediatric patients. An ongoing paediatric pilot, clinical trial (NCT04684238 and NCT05867472), will further increase our understanding of the feasibility and safety of using inhalational volatile gases for sedation in critically ill paediatric patients.
Practice Implications
Our results confirm that using the Deltasorb® Series 1000 scavenging system with volatile anesthetic agents and active humidification remains an efficient scavenging system with exposure levels maintained below occupational health and safety limits. The use of active humidity can contribute a substantial absorbent load on scavenging systems. We would recommend frequent changes (e.g., every second day if used in tandem) to ensure that capacity remains available for inhaled anesthetic absorption. The combination of active scavenging, using canisters in tandem, and active scavenging as a fail-safe ensures that paediatric critical care providers are working in a safe environment.
Acknowledgements
The authors would like to thank: Blue Zone Technologies for providing Deltasorb® Series 1000 scavenge canisters and performing analysis of the extracted volumes of the canisters tested; Matthew Williston for creating a brief report of the analysis, Winnie Seto, R.Ph, PharmD for her review of the manuscript, and University Health Network (Toronto General Hospital RT Department) for kindly loaning their Portable Ambient Analyzer.
Contributors
All authors contributed to the conception or design of the work, the acquisition, analysis, or interpretation of the data. All authors were involved in drafting and commenting on the paper and have approved the final version.
Funding
AJ, MS, BC, SLG, NKM have ongoing clinical trials examining the effects of inhaled anesthetics on patient and health system outcomes in adult and paediatric critically ill adults that are funded by the Canadian Institute of Health Research.
Conflict of Interest
Blue Zone Technologies, Canada did perform the desorption procedure and provided analysis of volatile and water vapor capture. All data related to occupational health and safety was undertaken through our office of Occupational Health and Safety, and neither Sedana Medical nor Blue Zone contributed to testing nor were aware of the results.
Ethical Approval
This study was reviewed by The Hospital for Sick Children IRB and deemed to meet the criteria for exemption.
AI statement
The authors confirm no generative AI or AI-assisted technology was used to generate content.