Introduction
Chronic obstructive pulmonary disease (COPD) is a multi-factorial and progressive chronic respiratory noncommunicable disease. The worldwide prevalence of COPD is approximately 9% in men and 6% in women,1 and it is the leading cause of mortality due to chronic respiratory diseases.2 Individuals with COPD are often affected by one or more comorbidities,3 increasing their risk of disability, impaired quality of life, and mortality.4
The natural course of COPD is marked by acute episodes of exacerbation of respiratory symptoms. Dyspnea and productive coughing with production of sputum are common respiratory symptoms in individuals with COPD. These symptoms result from a chronic airflow limitation caused by airway and/or alveolar abnormalities associated with particles or harmful gases, creating an increased inflammatory response of the lung and recurrent chest infection.5 Recurrent exacerbations are common in patients with COPD due to the decline of lung function and are the most common cause of medical hospital re-admission,6 which results in high socioeconomic burden.7 These health and societal implications highlight the need for cost-effective intervention and secondary prevention strategies to reduce the risk of developing or exacerbating the symptoms of COPD. Therefore, patients with acute exacerbations of COPD should be followed with pulmonary rehabilitation after hospital discharge to decrease the risk of rehospitalizations8 and significantly reduce the associated costs.9 A myriad of exercise-based programs - including aerobic exercise, resistance exercise, water-based exercise, Yoga and Tai Chi - have shown to be effective in patients with COPD to enhance exercise capacity and function, reduce COPD-related symptoms and impairments, while also improving their health-related quality of life.10–15 The engagement in physical activity and exercise-based interventions has proven to be a cost-effective strategy with relevant gains in quality-adjusted life years (QALYs).16,17 Therefore, it is clear that medication and traditional breathing exercises should always be complemented with exercise-based interventions in patients with COPD.
Due to obstructive airflow impairments that increase airway resistance, patients with COPD have difficulty generating the necessary negative pressure to draw air into the lungs. Consequently, there is an increased workload of the diaphragm that can result in fatigue and reduced efficiency in breathing.18–21 Patients with COPD have a rapid and shallow breathing pattern characterized by dynamic hyperinflation and air trapping,22 with breath-to-breath fluctuations.23 This breathing pattern places extra effort on the accessory respiratory muscles due to the inefficiency of the diaphragm and the intercostal muscles in providing adequate ventilation.24 This mechanism can lead to weakness and fatigue of respiratory muscles that often result in dyspnea that is a hallmark symptom of patients with COPD.
Pulmonary rehabilitation programs implement exercises that promote diaphragmatic breathing aiming to improve thoracic mobility, enhance diaphragm and abdominal movement, and thus decrease the effort placed at the respiratory muscles of the ribcage.25 A Pilates exercise program training can help to complement these traditional pulmonary rehabilitation programs, as it provides a more controlled setting of deep breathing exercises, which differ from diaphragmatic breathing exercises commonly seen in pulmonary rehabilitation. Within each set of deep breathing exercises, the individual keeps the abdomen pulled in as they actively contract the transverse abdominal and pelvic floor muscles.26
There are several mechanisms of a Pilates exercise program that can contribute to clinically relevant improvement in individuals with COPD. Pilates can improve the strength of expiratory muscles by increasing the strength and flexibility of the core and lumbopelvic muscles,27,28 improved flexibility and enhanced movement patterns in the ribcage29 and upper and lower limbs.,27,28 which are of upmost importance in patients with COPD.27,30 The controlled concentric and eccentric breathing techniques employed during Pilates training can boost lung capacity and improve the functionality of the deeper abdominal muscles (transversus abdominis, lumbar multifidus, and the respiratory and pelvic diaphragms).31 The respiratory and breathing techniques employed during the Pilates exercise program can thus contribute to the improvement of strength of respiratory muscles. By improving the strength of respiratory muscles, a Pilates exercise program can prevent wasting respiratory muscles, improve respiratory function, and increase resistance to fatigue of respiratory muscles, thus contributing to decrease common COPD-related symptoms, most notably chronic dyspnea.
Pilates exercise programs have been gaining popularity and have shown positive effects in a wide range of clinical conditions32; however, there is insufficient evidence of the potential positive effects in individuals with COPD.26 Hence, our main goal was to compare the benefits home-based pulmonary rehabilitation with or without a Pilates exercise program after an initial three months of traditional community-based pulmonary rehabilitation in individuals with COPD. Lung function and strength of respiratory muscles were defined as primary outcomes, while cardiac, physical function, and the number of exacerbation episodes were defined as secondary outcomes. We hypothesized that the group with the Pilates exercise program would significantly improve lung function and strength of respiratory muscles as compared to the control group with home-based exercises.
Methods
The study was approved by the Institutional Ethical Committee (IRB number: ERS NORTE, study ID T667, No.37/2017). All participants signed an informed consent form before enrolling in this study, and all procedures were conducted according to the Declaration of Helsinki.
Design and study population
This study was a non-randomized clinical trial, in which the participants were assigned to the groups by the researchers.
From July 2018 to May 2021, a general practitioner working in the National Health Service (in the north of Portugal) invited adult individuals (≥ 18 years old) with COPD to participate in the current study. Participants of both sexes with COPD and presenting stable symptoms for the last month were eligible for this study. The recruited participants were evaluated according to the updated Global Initiative for Chronic Obstructive Lung Disease (GOLD) system criteria,33 and only those classified as GOLD B were included in this study. The goal in using this criterion was to homogenize the symptoms of COPD of the included participants.
The exclusion criteria were: (i) over 85 years old; (ii) history of acute cardiac or respiratory events in the last month; (iii) any medical condition that would limit their exercise tolerance (severe cardiac, musculoskeletal, neuromuscular conditions, or recent surgery); (iv) psychiatric conditions or cognitive deficits; (v) history of neoplasia or immunologic diseases; or (vi) unwillingness to attend the community-based exercise program.
Sample size
An a priori sample size calculation was conducted using the Software G*Power (Version 3.1.9.2, Kiel, Germany) based on the results of a previous randomized controlled trial,34 using the forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio (FEV1/FVC) as the reference outcome after six months of pulmonary rehabilitation.
For the sample size calculation, a power (α) of 95% was considered to detect statistically significant differences (p<0.05) using a two-tailed independent Mann-Whitney test.
A total of 20 participants (10 per group) were needed to find statistically significant power; however, considering that dropout rates in pulmonary rehabilitation interventions are usually under 30%,35 a total of 14 participants were assigned to each group.
Procedures
Participants were divided into control and intervention groups in a non-randomized way. Enrollment of participants was made consecutively within the same community and no purposeful procedures were done for between-group sample matching. We employed a consecutive participant enrollment (within the same group) to allow a temporal matching of participants (schedule-wise) to form groups of community-based classes. All participants (of both groups) followed the same traditional community-based pulmonary rehabilitation program for the first three months of the study. Following this initial intervention, both groups were instructed to perform a set of recommended home-based exercises for the remaining six months of the study (from the 3rd to the 9th month). While the control group was restricted to the home-based exercises, the intervention group also performed a Pilates exercise program in addition to the home-based exercises.
Both groups were evaluated at baseline and at the end of the traditional community-based pulmonary rehabilitation (three months). Subsequently, both groups were reevaluated at the sixth and ninth month from baseline.
Intervention
Traditional community-based pulmonary rehabilitation
An initial community-based pulmonary rehabilitation intervention was equally implemented in both groups twice a week (60 minutes each) for the first three months. Intervention classes (from 4 to 12 participants per class) were provided by the same trained physiotherapist within the community context.
The traditional pulmonary rehabilitation program was composed of a warm-up, aerobic exercise, resistance strengthening, and cooldown.
The warm-up, which lasted ten minutes, consisted of joint mobility and light stretching exercises of the spine, upper, and lower limbs and was combined with breathing exercises.
The aerobic exercise consisted of walking for 20 minutes at an effort of 4–6 on the perceived dyspnea/fatigue on the modified Borg scale.36 Participants started with a walking speed that was perceived as 5 on the modified Borg scale. They were then asked every five minutes about their perceived dyspnea/fatigue, and if it were above 6, the walking speed was decreased until reaching 5 on the modified Borg scale.
Resistance exercises lasted 20 minutes and included the major upper muscle groups (biceps, triceps, deltoid, pectoral major and latissimus dorsi), major lower limb muscle groups (knee flexors and extensors and hip abductors and adductors), the flexor and extensor trunk, and lower back muscles. The exercises for each muscle group changed every three weeks during the three months of the exercise program. Strengthening exercises (2 sets of 10 to 12 repetitions) were performed by the participants using free weights and ankle weights. A load that evokes fatigue after 10 to 12 repetitions was appropriated. The exercise dosage was increased over time (the so-called overload) to facilitate improvements in muscular strength and endurance. This increase occurred when an individual performed the current workload for 1 or 2 repetitions over the desired number of 12, on two consecutive training sessions.36
The ten-minute cooldown included balance training comprising static and dynamic exercises in an upright position in addition to the same exercises used in the warm-up.
At the beginning of the study (baseline), participants in both groups were encouraged to do a set of recommended home-based exercises in order to complement the in-person classes (see Supplement 1). Following the initial three months, both groups were instructed to keep the same home-based exercises twice a week for another six months. Participants were required to keep a home-based exercise diary during the entire duration of the study. At the end of each week, they were contacted to determine if they had performed the recommended home-based exercises. If compliance was under 80% (controlled at each three months), the participant was excluded from the follow-up.
Exercise program based on Pilates principles
The intervention group received a Pilates exercise program for six months after the initial 3-months of traditional pulmonary rehabilitation. The Pilates exercise program was led and supervised by the same physiotherapist and implemented twice a week for 45 minutes per session. The Pilates exercise program was divided into two stages: one first stage (4th to 6th months) with a set of more simple and less challenging exercises to accommodate the participants to this type of exercise and a second stage (7th to 9th months) with a new set of more challenging exercises to allow progression while increasing the difficulty of exercises.
The first Pilates session (beginning of the 4th month) started with five basic and less physically demanding exercises (see Figure 1A-E). Each of the five exercises required two sets of ten repetitions. When performing unilateral exercises, the exercise was completed eight times for each side. The rest between exercises was 60 seconds. Exercises were first demonstrated by the physiotherapist and then repeated by participants. The importance of steady and controlled breathing cycles (inhalation and exhalation) during the pilates exercises were highlighted and shown by the physiotherapist.
Progression in the difficulty of exercises was made at the participant level and occurred when the participant demonstrated that one of the five previous exercises was not sufficiently challenging (below 4 points in the modified Borg scale, asked during the rest time between exercises) and shown that the exercise was done with the proper technique and without compensatory movements. For progression, there were four additional and more challenging exercises (see Figure 1F-I). However, if the participant was unable to perform the new exercise, the exercise was regressed to the previous, more basic one. In the following week, the technique with the new exercise was retested. After the first three months of the Pilates exercise program, the set of exercises changed to allow variability and provide more challenging exercises. Five new and more challenging exercises (see Figure 2A-E) were added with four additional exercises for progression (see Figure 2F-I). At this second stage, each exercise was completed for 2 sets of 12 repetitions. The same progression and regression rules were applied for this new set of exercises. However, if any participant could not tolerate the difficulty of any of the new exercises, they regressed to the exercises of the set of the first three months of the Pilates exercise program, and then progressed as tolerated. The full exercise Pilates protocol is described in Supplement 2.
Outcome measures
At baseline, we collected the sociodemographic and biometric data of all participants. Lung function and strength of respiratory muscles were defined as primary outcomes while cardiac, physical function, and the number of exacerbation episodes were defined as secondary outcomes.
Lung function
Lung function was evaluated by spirometry (MicroLoop® and MicroLab®, Care Fusion, Basingstoke, UK): FVC, FEV1, and FEV1/FCV ratio. The MicroLoop® spirometer has been proven to be accurate and precise to measure lung function in the population with COPD.37 The respiratory rate (cycles per minute [CPM]) and oxygen saturation (% of SpO2) were also collected. Each metric was repeated five times and the highest value was used.
Strength of respiratory muscles
The strength of inspiratory muscles was measured by a digital mouth respiratory pressure meter (MicroRPM®, Care Fusion, Basingstoke, UK) to determine the maximal inspiratory and expiratory pressure. The MicroRPM® is considered the gold standard device to measure the strength of respiratory muscles and has shown high intra- and inter-rater reliability.38 Each metric was repeated five times and the highest value was used.
Cardiac function
Cardiac function was assessed by the systolic and diastolic arterial blood pressure and heart rate using a digital blood pressure cuff device (Omron M6 Comfort, Omron Healthcare Ltd., Kyoto, Japan). This device has proven to be a valid and reliable measure of blood pressure according to defined international protocol.39 Each metric was repeated five times and the highest value was used.
Physical function
Physical function comprised assessment of muscle strength, exercise-induced fatigue, and balance. Muscle strength was evaluated using a hand-held dynamometer (microFET2, Hoggan Health, Salt Lake City, Utah) to measure (in kilogram-force [KgF]) the isometric quadriceps and handgrip strength. The microFET2 device provides excellent intra- and inter-rater reliability to measure quadriceps strength in patients with COPD.40 For quadricep strength, participants were seated with the knee flexed at approximately 90 degrees and were asked to perform knee extension. The dynamometer was placed distally to the anterior leg (5 cm above the lateral malleolus). The handgrip strength was measured with the participant seated and with the elbow unsupported and flexed at 90 degrees. The participant was asked to grip the dynamometer as hard as possible. Each strength metric was repeated three times and the highest value was used.
Exercise-induced fatigue was evaluated with the one-minute sit-to-stand and the six-minute walking test (6MWT). Both tests are reliable and provide a valid measure to estimate functional exercise performance in COPD patients.41,42
Balance was measured using the abbreviated version of the Balance Evaluation Systems Test (BESTest): the Brief BESTest.43 The Brief BESTest consists of six items (scored from 0 to 3 points each) for a total of 24 points. Lower scores indicate more severe balance impairment. The Brief BESTest is a valid, reliable, and valuable tool to identify the balance status in patients with COPD.44
Exacerbations
The number of respiratory-related exacerbation episodes in the preceding three months was collected at each follow-up endpoint. Exacerbation episodes were defined as acute worsening of respiratory symptoms (e.g., dyspnea, increased cough frequency, or severity and increase of sputum production or change of color) requiring additional treatment or hospitalization.
Statistical analysis
The software IBM SPSS version 28.0 (Statistical Package for the Social Sciences, Inc.; Chicago, IL) was used for all statistical analyses. Gaussian distribution was assessed through the Kolmogorov–Smirnov test and the skewness and kurtosis absolute values, which displayed a non-normal distribution in most of the included variables. Due to the non-Gaussian distribution and the small sample size, it was decided to conduct non-parametric statistics. Participants lost to follow-up were included in assessments where they were available and discarded in the evaluation endpoints were lost. The continuous variables are described using median and interquartile range, and categorical variables as count and frequency (%). For the hypothesis test, a P value of 0.05 was used to determine statistical significance.
Between groups statistical differences were compared using the Mann-Whitney U tests. The x2 test was used to compare categorical variables between groups as well as the exact Fisher test (when applicable). Within-group statistical differences were compared using the one-way repeated measures Friedman test (for overall changes throughout the follow-up) with the post hoc Wilcoxon signed-rank tests for pairwise comparisons between follow-up endpoints.
Results
Characteristics of included participants
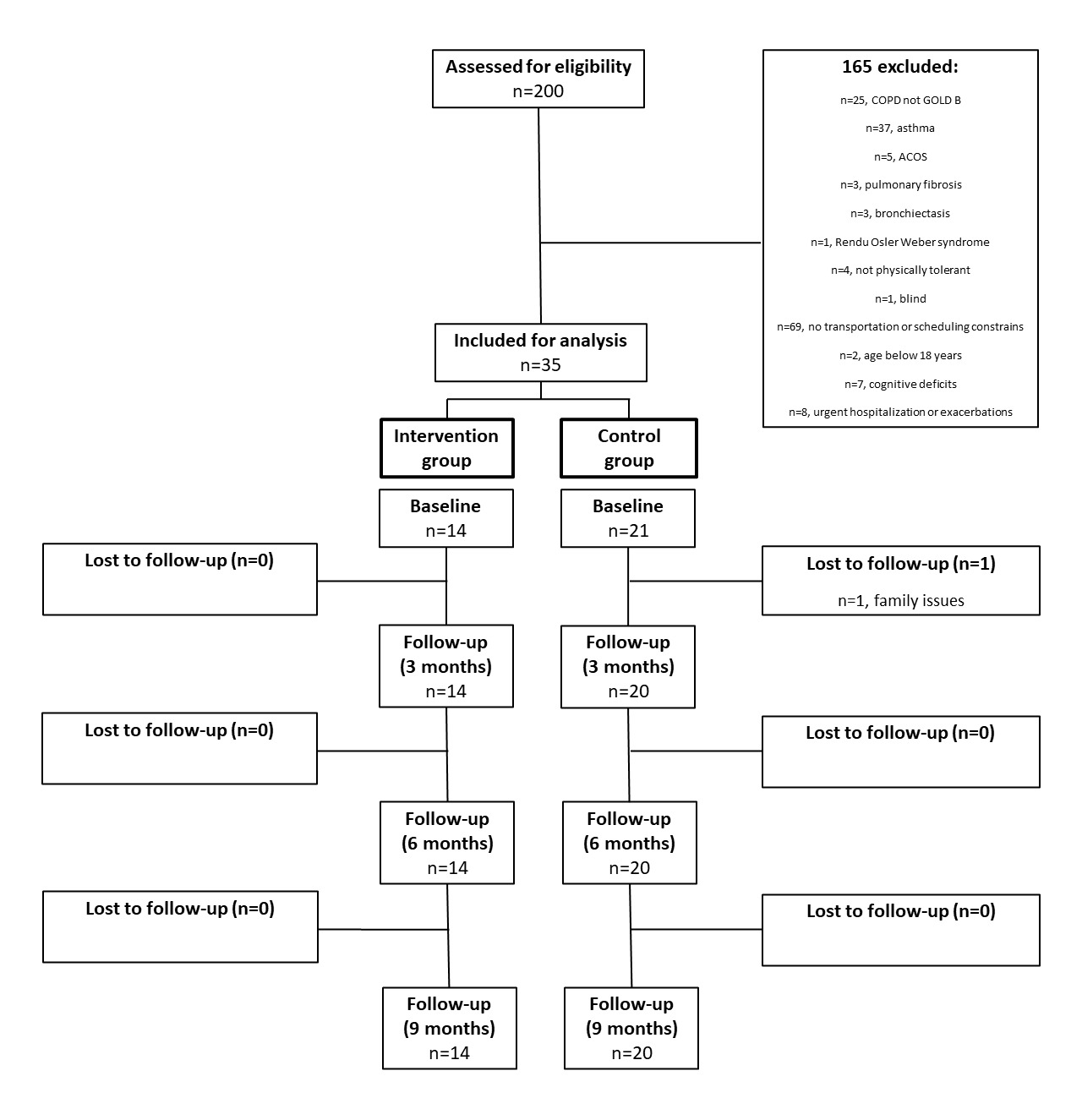
Of the 200 participants screened for inclusion in this study, 140 were initially excluded for not fulfilling the eligibility criteria. The most common reasons for exclusion were non-COPD respiratory diseases and the inability to participate in the study due to transportation or scheduling constraint. Moreover, further 65 participants were excluded as they presented other COPD grades besides GOLD B. A total of 35 participants were eligible and enrolled in the study. Each participant was allocated to either the intervention (n=14) or control (n=21) group. One patient in the control group was lost at follow-up at the three-month follow-up (Figure 3).
The baseline demographic and biometric characteristics were homogenous between the groups (Table 1). The baseline GOLD characteristics (stage and symptoms) were also mostly homogenous between the groups. Only the rate of exacerbations was statistically different, with the intervention group showing a significantly superior rate of two or more exacerbations in the last year (78.6% vs 0.0%).
Primary outcomes
The respiratory rate (CPM) and oxygen saturation (% SpO2) were not statistically different between the groups at baseline or during any follow-up end-point (p>0.05); neither were the within-group changes throughout the nine months of the study (Table 2).
The FEV1 (L) was not significantly different between the groups, and although it improved from baseline to three months in the control group by 0.5 L (p=0.002), the effect deteriorated at nine months from the baseline values (p=0.021). Both groups showed a non-significant improvement of FEV1 (%) at three months (change of 10.5% in the intervention group and 9% in the control group), but with significantly superior values in the control group (p=0.039). The improvement of FEV1 (%) deteriorated in both groups at nine months (decrease of 7.5% and 13% in in intervention and control group, respectively). The FCV (L) was comparable between the groups at baseline and throughout the follow-up, but with a statistically significant decrease of 0.3 L at nine months in the control group (p=0.001). The FVC (%) was significantly superior in the control group at baseline (77.3% and 99.0% at intervention and control group, respectively; p=0.002), but it deteriorated significantly throughout the follow-up until reaching similar values to the intervention group. The FEV1/FVC (%) showed a significant 29% increase in the control group at three months (p=0.001), which was maintained at six and nine months, always superior compared to the intervention group (p<0.005).
The strength of inspiratory and expiratory muscles increased significantly at three months in both groups (p<0.05). However, the maximal inspiratory pressure was significantly superior by 37 cm H2O in the intervention group at nine months for maximal inspiratory pressure (p=0.005). Likewise, the maximal expiratory pressure was also significantly superior in the intervention group by 40.5 cm H2O at six months and by 45 cm H2O at nine months (p=0.027 and p<0.001, respectively). Although maximal inspiratory and expiratory pressure showed a marginal improvement at three months in the control group (p<0.05), both measures displayed a significant decrease at nine months (p<0.001).
Secondary outcomes
The systolic and diastolic blood pressure showed significant variant patterns across the nine months of the study. Despite statistically significant differences between groups at nine months (p=0.009 and p<0.001, respectively; Table 3), the systolic blood pressure was within Stage 1 of hypertension in both groups. The diastolic blood pressure was within the range of normal values for the control group and at the lowest range of Stage 1 of hypertension (80 mm Hg) for the intervention group.
Isometric quadricep strength showed an improvement in both groups at three months (13.7 KgF and 6.3 KgF in the intervention and control group, respectively). However, the improvement deteriorated by 4.1 KgF until the ninth-month follow-up in the control group (p<0.001). Although the improvement was superior in the intervention group, it was not statistically significant in any of the follow-up endpoints (p>0.05). Likewise, the handgrip strength improved in both groups but started to deteriorate at six months in the control group (p=0.001). Handgrip strength was not statistically significant between groups in any of the follow-up endpoints (p>0.05).
Exercise-induced fatigue (one-minute sit-to-stand and 6MWT) was comparable between groups at baseline (Table 3). However, after an improvement at three months in the control group (p<0.05), both exercise-induced fatigue metrics deteriorated over time (p<0.001). The intervention group showed a significantly higher number of repetitions at six months (more 5.5 repetitions, p=0.027) and nine months (more 4.5 repetitions, p=0.004) in the one-minute sit-to-stand test. Similarly, the intervention group outperformed the control group in the 6MWT, with more 92.5 meters at six months (p=0.047) and more 178.5 meters at nine months (p<0.001).
Balance (Brief BESTest) was comparable between groups at baseline and after the initial three months of intervention (p>0.05), but the intervention group showed superior balance scores at the six months (two points more, p=0.003) and nine months (four points more, p<0.001; see Table 3).
Although the intervention group showed a higher number of exacerbations, the rate was not statistically different between the groups at any follow-up endpoint (p>0.05; see Supplement 3).
Discussion
The main finding of this prospective cohort study showed that adding a six-month Pilates exercise program to a three-month pulmonary rehabilitation program resulted in superior strength of inspiratory and expiratory muscles when compared to the group that only received the initial three months of pulmonary rehabilitation. Moreover, the changes at follow-up in the maximal inspiratory pressure surpassed the minimal clinically important difference (MCID),45 showing that the improvement was large enough to be perceived as clinically meaningful by the patient. This finding highlights a potential role that a Pilates exercise program may play on improving the strength of respiratory muscles, which is clinically relevant, as the respiratory muscles play a crucial role in the diaphragmatic function in patients with COPD. Our findings are in line with other studies that show improved strength of respiratory muscles after the implementation of a Pilates exercise program for different pathological conditions.27,30 Therefore, physiotherapists may consider complementing their traditional pulmonary rehabilitation program with a structured Pilates exercise program.
Interestingly, the positive findings on the strength of respiratory muscles were not translated into consistent improvement of lung function measured by spirometry. Most lung function outcomes showed a similar behavior between the intervention and control group throughout the nine-month follow-up. This finding may be explained by the fact that the participants in the control group also followed the same recommended set of home-based exercises that may have contributed to the maintenance of improvement in lung function after the initial three months of traditional pulmonary rehabilitation program. In fact, the FEV1/FVC (%) was significantly superior in the control group at six- and nine-month follow-ups; however, this may be a confounding result because the FEV1/FVC (%) was already significantly superior in the control group after the initial three months of traditional pulmonary rehabilitation program (before the implementation of the Pilates exercise program). This may partially explain why this outcome was also significantly superior at the six- and nine-month follow-up. Notwithstanding the lack of superiority in lung function of the intervention group at follow-up, the significantly superior positive impact on the strength of respiratory muscles is sufficient to justify the addition of a Pilates exercise program. Indeed, many lung-related ventilatory parameters can be improved with Pilates-based breathing exercises.26
The intervention group also showed superiority in exercise-induced fatigue and balance at follow-up. Improving exercise-induced fatigue is an important outcome in patients with COPD because the resistance to fatigue of these patients is often compromised due to dyspnea-related symptoms and to a smaller proportion of type I fibers that are more resistant to fatigue.46 The improvement in the intervention group is clinically meaningful, as it surpassed the MCID for both the 6MWD41 and number of chair raises.47 Moreover, the between-group differences for the 6MWD at the nine-month follow-up (178.5 meters) are larger than the 41 meters that is typically reported when comparing traditional pulmonary rehabilitation interventions to control groups.48 These metrics, such as the number of chair raises, are important in evaluating the tolerance of fatigue in patients with COPD and have been associated with symptoms such as dyspnea.49 Improvements in the 6MWD and other related to exercise-induced fatigue are also seen in other studies that implemented pilates in other pathological conditions.50,51 Likewise, improving balance in patients with COPD is important because impairments and decline in balance are more pronounced in these patients due to exercise intolerance and dyspnea52,53 as well as other physiological factors such as muscle weakness54 and somatosensory deficits.55 The Pilates exercise program may have contributed to improved balance through better muscle control of the deeper abdominal muscles.31 In line with exercise-induced fatigue, the changes at follow-up in the intervention group for the Brief BESTest also surpassed the MCID.56 Similar findings on balance after a Pilates exercise program have also been reported for many other populations.31,32,57
Isometric quadricep and handgrip strength, despite not being statistically significant between groups, indicated that median improvement was higher in the intervention group. Indeed, while both strength metrics stayed relatively constant for the control group, they showed a relevant improvement in the intervention group with the isometric quadricep strength more than doubling at six months and the handgrip strength being almost twofold that of the control group at nine months follow-up. Quadricep strength plays an important role in patients with COPD in opposing exercise tolerance58 and has an association with longer survival.59 The improvements in isometric strength surpassed the MCID at the six- month follow-up and were almost fourfold the MCID at the nine-month follow-up.45 Similarly, handgrip strength is important in patients with COPD as it is impaired during exacerbation episodes60 and is associated with dyspnea symptoms and functional exercise capacity.61 Similar improvements in quadricep muscle strength have been reported in other studies that have implemented a Pilates exercise program for other pathologic populations.32,57 The lack of between-group statistical significance in these two strength metrics at follow-up may have been the result of a type II error, which is discussed at more length in the limitations section.
The strengths of our study are the implementation of a structured exercise program during 9 months with a high degree of adherence, which is often challenging in a cohort of COPD patients. Moreover, our study investigates the effects of a particularly relevant and insufficiently researched type of exercise (Pilates) in patients with COPD, showing that the theoretical benefits of pilates in lung and respiratory function in individuals with COPD can translate into measurable and clinically relevant improvements. Future research should improve upon our research design by implementing a randomized control trial comparing the effects of Pilates exercise against a control group with usual care and expand into other subgroups of this population, with other grades of COPD other than GOLD B.
This study has some limitations that need to be acknowledged. Despite being above the a priori sample size calculation (at least ten per group), our sample size is still small. Moreover, the intervention group had a lower sample size than the control group due to the exclusion of some patients who were not classified as GOLD B. A total of 25 participants with grades other than GOLD B completed the entire study, but we decided to exclude these from analyses to prevent selection bias. Moreover, subgroups based on GOLD grades were not possible because this would have resulted in very small groups and low representativeness (external validity). These limitations led to a lower statistical power in analyses and may have resulted in a type II error in not finding statistical differences when the between-groups differences were clinically relevant (e.g., for isometric quadriceps and handgrip strength). Another limitation was the lack of randomization and blinding of outcome assessors which may increase the risk of selection and detection bias, respectively. Lastly, the control group was not left without intervention throughout the follow-up period, as it would have been unethical to leave these patients without any guidance. Therefore, the participants of the control group were encouraged to continue performing the recommended home-based exercises throughout the follow-up period. To mitigate a potential performance bias, the intervention group was also encouraged to follow the same home-based exercise program, and adherence to the home-based exercises was monitored to assure that it was over 80% in both groups. The home-based exercises seem not to have yielded an effect in the control group during the follow-up because muscle strength and tolerance to exercise-induced fatigue deteriorated in the control group at the six- and nine-month follow-ups. However, it must be acknowledged that the intervention group followed a structured exercise regimen (Pilates) as compared to solely unsupervised home-based exercises and that results may have arisen from the effects of following a structured exercise program14 (regardless of being pilates or other type of exercise). Indeed, similar improvements have been observed in patients with COPD following other types of structured exercise programs.10–15 Moreover, the superior effect on the intervention group could also be (at least partially) attributed to the potential effect of additional exposure to exercise-based intervention. After the initial 3 months of traditional pulmonary rehabilitation, while the control group followed home-based exercises for 6 months, the intervention group received a structured Pilates-based exercise program twice week in addition to the home-base exercises.
Conclusion
The intervention group, in which a six-month Pilates exercise program was added to an initial three-month pulmonary rehabilitation program, showed superior strength of inspiratory and expiratory muscles, higher resistance to exercise-induced fatigue, and improved balance in patients with COPD as compared to the control group that only performed the initial three-month pulmonary rehabilitation program. These findings underpin the relevance and possibility of complementing the traditional pulmonary rehabilitation with a structured Pilates exercise program to enhance and/or maintain the improvement of pulmonary rehabilitation.
Funding
Not applicable.
Competing Interests
All authors have completed the ICMJE uniform disclosure form and declare no conflict of interest.
AI statement
The authors confirm no generative AI or AI-assisted technology was used to generate content.
Ethical Declarations
The study was approved by the Institutional Ethical Committee (IRB number: ERS NORTE, study ID T667, No.37/2017).